- Medspa Essentials
- Aestheticians
- Apparel
- Clinic Furniture
- Devices
- Pharmaceutical & Medical Supplies
- Safety Supplies
- Training & Services
- Vendors
- Acara Partners
- Aesthetic Next
- Aesthetic Record
- American Med Spa Association
- Bonsai
- CEDR HR Solutions
- Colorescience
- CosmoGlo
- Derma Made
- Docovia
- Eden Skin And Body
- FACE Med Store
- KA Ring Designs
- LIFTIE Aspirator
- Massage Tools
- Merit Pharmaceutical
- National Medical Directors
- Post Love Skincare
- Rana Kennelley
- Source One Beauty
- SyringeRack
- The Aesthetic Immersion
- Velez by Vesna
- We Treat
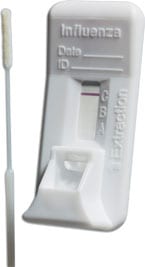
Orasure Quickflu Rapid Flu A & B Test, 22 Tests Box 013602705375
$318.99
Orasure Quickflu Rapid Flu A & B Test, 22 Tests/Bx
# 1001-0320
Non-Returnable
The OraQuick® HCV test is FDA approved for detecting HCV antibodies in fingerstick and venipuncture whole blood. Our simple platform enables healthcare providers to deliver an accurate diagnosis in 20 minutes.
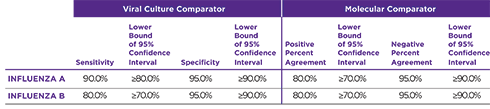
SENSITIVITY TO MEET YOUR NEEDS
OraSure QuickFlu® is an FDA cleared, CLIA-waived in vitro rapid qualitative test for the detection and differentiation of influenza type A and type B. An easy nasal swab procedure provides accurate results in as little as 10 minutes.
Orasure QuickFlu® delivers highly accurate results for flu types A and B and meets FDA’s new standards of performance.
- Proven analytical sensitivity across 51 different flu strains including CDC’s 2019-2020 flu strain panels
- Exceptional correlation to culture/PCR
PROVEN PERFORMANCE THAT MEETS FDA’S RECLASSIFICATION RULES
OraSure QuickFlu® exceeds the FDA’s reclassification requirement minimums for RIDTs.
- As of February 13, 2017, all RIDTs must meet the minimum acceptance performance criteria and comparator method for antigen based RIDTs. Tests that do not meet the new requirements cannot be used.
- Analytical reactivity testing must be performed on contemporary influenza strains and newly emerging strains in the event of an emergency.
ENSURES RESPONSIVE STANDARD OF CARE
-
- Simple, easy-to-use procedure with built-in control line
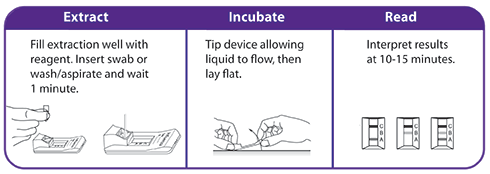
- Includes a flocked swab that optimizes sample collection and release resulting in superior performance
- American Society of Microbiology says, “NP flocked swabs collect better samples than routine NP swabs”
- Nasal swab collection – comfortable for the patient and simple for the HCP with no ancillary supplies required
- Ready-to-use reagents with room temperature storage
- Rapid testing that maximizes results
TEST AT THE POINT OF CARE
OraSure QuickFlu® is ideal for testing at the point of care in…
- Laboratories
- Emergency Rooms
- Physician Offices
- Public Health Facilities and Clinics
REIMBURSEMENT INFORMATION
CPT Codes
87804 QW- Influenza A Detection
87804-59- Influenza B Detection
SKU
1001-0320
Categories Lab Supplies, Pharmaceutical & Medical Supplies, Pharmacy
Tags antibodies, diagnose, diagnostic, flu test, HCV, Medical Supplies, Orasure, Quickflu, rapid, Rapid Flu, test box
Brand: OraSure QuickFlu
Additional information
| Weight | 2.27 kg |
|---|---|
| Dimensions | 20.32 × 10.16 × 20.32 cm |
Be the first to review “Orasure Quickflu Rapid Flu A & B Test, 22 Tests Box 013602705375” Cancel reply
You must be logged in to post a review.
-
$9.99 – $272.00Price range: $9.99 through $272.00Sold By: Merit Pharmaceutical
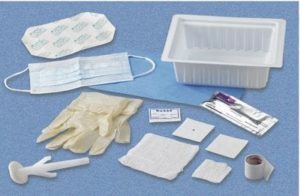
Central Line Tray With ChloraPrep One-Step Applicator 078121197500
$9.99 – $272.00Price range: $9.99 through $272.00 Select options -
$1.00 – $50.00Price range: $1.00 through $50.00Sold By: Merit Pharmaceutical
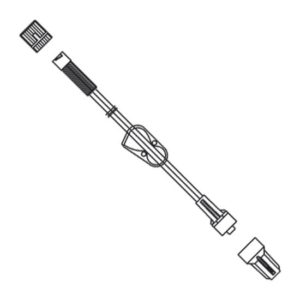
Extension Set, Minibore, 7”, Meritset 036001170000
$1.00 – $50.00Price range: $1.00 through $50.00 Select options -
$66.50
-
$1.99 – $62.50Price range: $1.99 through $62.50Sold By: Merit Pharmaceutical
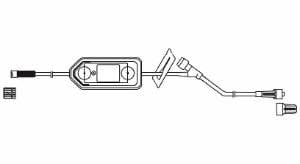
Extension Set, Regular Bore, 7”, Meriset 088713723000
$1.99 – $62.50Price range: $1.99 through $62.50 Select options
Additional information
| Weight | 2.27 kg |
|---|---|
| Dimensions | 20.32 × 10.16 × 20.32 cm |
Be the first to review “Orasure Quickflu Rapid Flu A & B Test, 22 Tests Box 013602705375” Cancel reply
You must be logged in to post a review.
Reviews
There are no reviews yet.
-
$9.99 – $272.00Price range: $9.99 through $272.00Sold By: Merit Pharmaceutical
Central Line Tray With ChloraPrep One-Step Applicator 078121197500
$9.99 – $272.00Price range: $9.99 through $272.00 Select options -
$1.00 – $50.00Price range: $1.00 through $50.00Sold By: Merit Pharmaceutical
Extension Set, Minibore, 7”, Meritset 036001170000
$1.00 – $50.00Price range: $1.00 through $50.00 Select options -
$66.50
-
$1.99 – $62.50Price range: $1.99 through $62.50Sold By: Merit Pharmaceutical
Extension Set, Regular Bore, 7”, Meriset 088713723000
$1.99 – $62.50Price range: $1.99 through $62.50 Select options
Additional information
| Weight | 2.27 kg |
|---|---|
| Dimensions | 20.32 × 10.16 × 20.32 cm |
Be the first to review “Orasure Quickflu Rapid Flu A & B Test, 22 Tests Box 013602705375” Cancel reply
You must be logged in to post a review.
Reviews
There are no reviews yet.
-
$9.99 – $272.00Price range: $9.99 through $272.00Sold By: Merit Pharmaceutical
Central Line Tray With ChloraPrep One-Step Applicator 078121197500
$9.99 – $272.00Price range: $9.99 through $272.00 Select options -
$1.00 – $50.00Price range: $1.00 through $50.00Sold By: Merit Pharmaceutical
Extension Set, Minibore, 7”, Meritset 036001170000
$1.00 – $50.00Price range: $1.00 through $50.00 Select options -
$66.50
-
$1.99 – $62.50Price range: $1.99 through $62.50Sold By: Merit Pharmaceutical
Extension Set, Regular Bore, 7”, Meriset 088713723000
$1.99 – $62.50Price range: $1.99 through $62.50 Select options
Additional information
| Weight | 2.27 kg |
|---|---|
| Dimensions | 20.32 × 10.16 × 20.32 cm |
Be the first to review “Orasure Quickflu Rapid Flu A & B Test, 22 Tests Box 013602705375” Cancel reply
You must be logged in to post a review.
Reviews
There are no reviews yet.
-
$9.99 – $272.00Price range: $9.99 through $272.00Sold By: Merit Pharmaceutical
Central Line Tray With ChloraPrep One-Step Applicator 078121197500
$9.99 – $272.00Price range: $9.99 through $272.00 Select options -
$1.00 – $50.00Price range: $1.00 through $50.00Sold By: Merit Pharmaceutical
Extension Set, Minibore, 7”, Meritset 036001170000
$1.00 – $50.00Price range: $1.00 through $50.00 Select options -
$66.50
-
$1.99 – $62.50Price range: $1.99 through $62.50Sold By: Merit Pharmaceutical
Extension Set, Regular Bore, 7”, Meriset 088713723000
$1.99 – $62.50Price range: $1.99 through $62.50 Select options
Additional information
| Weight | 2.27 kg |
|---|---|
| Dimensions | 20.32 × 10.16 × 20.32 cm |
Be the first to review “Orasure Quickflu Rapid Flu A & B Test, 22 Tests Box 013602705375” Cancel reply
You must be logged in to post a review.
Reviews
There are no reviews yet.
-
$9.99 – $272.00Price range: $9.99 through $272.00Sold By: Merit Pharmaceutical
Central Line Tray With ChloraPrep One-Step Applicator 078121197500
$9.99 – $272.00Price range: $9.99 through $272.00 Select options -
$1.00 – $50.00Price range: $1.00 through $50.00Sold By: Merit Pharmaceutical
Extension Set, Minibore, 7”, Meritset 036001170000
$1.00 – $50.00Price range: $1.00 through $50.00 Select options -
$66.50
-
$1.99 – $62.50Price range: $1.99 through $62.50Sold By: Merit Pharmaceutical
Extension Set, Regular Bore, 7”, Meriset 088713723000
$1.99 – $62.50Price range: $1.99 through $62.50 Select options
Additional information
| Weight | 2.27 kg |
|---|---|
| Dimensions | 20.32 × 10.16 × 20.32 cm |
Be the first to review “Orasure Quickflu Rapid Flu A & B Test, 22 Tests Box 013602705375” Cancel reply
You must be logged in to post a review.
Reviews
There are no reviews yet.
-
$9.99 – $272.00Price range: $9.99 through $272.00Sold By: Merit Pharmaceutical
Central Line Tray With ChloraPrep One-Step Applicator 078121197500
$9.99 – $272.00Price range: $9.99 through $272.00 Select options -
$1.00 – $50.00Price range: $1.00 through $50.00Sold By: Merit Pharmaceutical
Extension Set, Minibore, 7”, Meritset 036001170000
$1.00 – $50.00Price range: $1.00 through $50.00 Select options -
$66.50
-
$1.99 – $62.50Price range: $1.99 through $62.50Sold By: Merit Pharmaceutical
Extension Set, Regular Bore, 7”, Meriset 088713723000
$1.99 – $62.50Price range: $1.99 through $62.50 Select options
Additional information
| Weight | 2.27 kg |
|---|---|
| Dimensions | 20.32 × 10.16 × 20.32 cm |
Be the first to review “Orasure Quickflu Rapid Flu A & B Test, 22 Tests Box 013602705375” Cancel reply
You must be logged in to post a review.
Reviews
There are no reviews yet.
-
$9.99 – $272.00Price range: $9.99 through $272.00Sold By: Merit Pharmaceutical
Central Line Tray With ChloraPrep One-Step Applicator 078121197500
$9.99 – $272.00Price range: $9.99 through $272.00 Select options -
$1.00 – $50.00Price range: $1.00 through $50.00Sold By: Merit Pharmaceutical
Extension Set, Minibore, 7”, Meritset 036001170000
$1.00 – $50.00Price range: $1.00 through $50.00 Select options -
$66.50
-
$1.99 – $62.50Price range: $1.99 through $62.50Sold By: Merit Pharmaceutical
Extension Set, Regular Bore, 7”, Meriset 088713723000
$1.99 – $62.50Price range: $1.99 through $62.50 Select options
Best selling
-
Easy Touch Insulin
$15.99 -
Non-Woven Sponges
$3.50 -
IV Start Kit Latex Free
$77.00
-
-
Sharps Container
$5.25
-
Related Products
Related Products
-
$113.00 – $127.50Price range: $113.00 through $127.50Sold By: Merit Pharmaceutical
BD Vacutainer Safety-Lok Blood Collection Set
$113.00 – $127.50Price range: $113.00 through $127.50 Select options -
Pressure Infusor Bag
Sold By: Merit Pharmaceutical$17.99 – $18.99Price range: $17.99 through $18.99Sold By: Merit PharmaceuticalPressure Infusor Bag
$17.99 – $18.99Price range: $17.99 through $18.99 Select options -
$430.00
-
$113.00 – $127.50Price range: $113.00 through $127.50Sold By: Merit Pharmaceutical
BD Vacutainer Safety-Lok Blood Collection Set
$113.00 – $127.50Price range: $113.00 through $127.50 Select options -
Pressure Infusor Bag
Sold By: Merit Pharmaceutical$17.99 – $18.99Price range: $17.99 through $18.99Sold By: Merit PharmaceuticalPressure Infusor Bag
$17.99 – $18.99Price range: $17.99 through $18.99 Select options -
$430.00
-
$650.00
$850.00Sold By: Rana KennelleyTwo Accredited Neuromodulator On-Line Training Courses
$650.00$850.00Add to cart -
$790.00
$990.00Sold By: Rana KennelleyTwo Accredited Facial Filler On-Line Training Courses
$790.00$990.00Add to cart -
$91.00
$113.99 -
Manual Bundle
Sold By: Acara Partners$1,250.00$1,750.00 -
Collins Cameo Reception Desk with 10 Color Options ($1 Shipping) Made in USA
Sold By: Source One Beauty$1,009.00$1,062.00Sold By: Source One BeautyCollins Cameo Reception Desk with 10 Color Options ($1 Shipping) Made in USA
$1,009.00$1,062.00Select options -
Collins Reve Concierge Desk with 10 Color Options ($1 Shipping) Made in USA
Sold By: Source One Beauty$1,031.50$1,086.00Sold By: Source One BeautyCollins Reve Concierge Desk with 10 Color Options ($1 Shipping) Made in USA
$1,031.50$1,086.00Select options -
Collins Reve Retail Display with 10 Color Options ($1 Shipping) Made in USA
Sold By: Source One Beauty$838.00$882.00 -
Collins Amati Retail Display with 10 Color Options ($1 Shipping) Made in USA
Sold By: Source One Beauty$792.50$834.00 -
$2,462.50
$2,592.00
-
$650.00
$850.00Sold By: Rana KennelleyTwo Accredited Neuromodulator On-Line Training Courses
$650.00$850.00Add to cart -
$790.00
$990.00Sold By: Rana KennelleyTwo Accredited Facial Filler On-Line Training Courses
$790.00$990.00Add to cart -
$91.00
$113.99 -
Manual Bundle
Sold By: Acara Partners$1,250.00$1,750.00 -
Collins Cameo Reception Desk with 10 Color Options ($1 Shipping) Made in USA
Sold By: Source One Beauty$1,009.00$1,062.00Sold By: Source One BeautyCollins Cameo Reception Desk with 10 Color Options ($1 Shipping) Made in USA
$1,009.00$1,062.00Select options -
Collins Reve Concierge Desk with 10 Color Options ($1 Shipping) Made in USA
Sold By: Source One Beauty$1,031.50$1,086.00Sold By: Source One BeautyCollins Reve Concierge Desk with 10 Color Options ($1 Shipping) Made in USA
$1,031.50$1,086.00Select options -
Collins Reve Retail Display with 10 Color Options ($1 Shipping) Made in USA
Sold By: Source One Beauty$838.00$882.00 -
Collins Amati Retail Display with 10 Color Options ($1 Shipping) Made in USA
Sold By: Source One Beauty$792.50$834.00 -
$2,462.50
$2,592.00
































Reviews
There are no reviews yet.