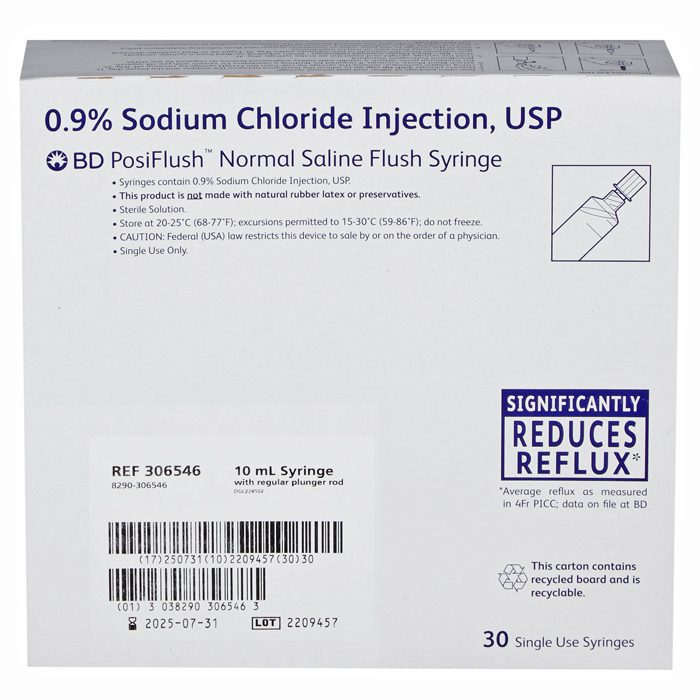
BD 306546 PosiFlush IV Flush Solution Sodium Chloride 0.9% Injection Prefilled Syringe 10 mL, 30/box
$26.95$30.00 (-10%)
BD 306546 Sodium Chloride 0.9% PosiFlush IV Flush Solution is designed for use in maintaining catheter patency by flushing IV catheters to prevent clotting and encourage flow. It is a sterile, isotonic solution for intravenous access. The solution contains 0.9% sodium chloride and helps prevent clotting and rid the catheter of particulate matter and bacterial contaminants. It is important to flush the catheter after any access or before changing any components, to prevent contamination and occlusion of the catheter lumen.
BD 306546 Sodium Chloride 0.9% PosiFlush IV Flush Solution is an essential intravenous flush for use in IV therapy. This solution flushes and cleans the lines and ports of IV systems for infants, adults and elderly patients before and after medication treatments. It helps eliminate bacteria and debris in the line and ensures proper infusion and yields a more accurate dosage of the medication. The solution is easily administered using a regulated flow and offers the merit of minimizing exposure of germs and organisms to the patient’s bloodstream. It is suitable for use in all health care settings and offers superior reliability to maximize efficacy.
BD 306546 PosiFlush IV Flush Solution Sodium Chloride, Preservative Free 0.9% Injection Prefilled Syringe 10 mL, 30/box
| Manufacturer # | 306546 |
| Brand | BD PosiFlush™ |
| Manufacturer | Becton Dickinson |
| Application | IV Flush Solution |
| Container Type | Prefilled Syringe |
| Dosage Form | Injection |
| Generic Drug Name | Sodium Chloride, Preservative Free |
| Storage Requirements | USP Controlled Room Temperature |
| Strength | 0.9% |
| Type | Intravenous |
| Volume | 10 mL |
BD 306546 Sodium Chloride 0.9% PosiFlush IV Flush Solution is designed for use in maintaining catheter patency by flushing IV catheters to prevent clotting and encourage flow. It is a sterile, isotonic solution for intravenous access. The solution contains 0.9% sodium chloride and helps prevent clotting and rid the catheter of particulate matter and bacterial contaminants. It is important to flush the catheter after any access or before changing any components, to prevent contamination and occlusion of the catheter lumen.
BD 306546 Sodium Chloride 0.9% PosiFlush IV Flush Solution is an essential intravenous flush for use in IV therapy. This solution flushes and cleans the lines and ports of IV systems for infants, adults and elderly patients before and after medication treatments. It helps eliminate bacteria and debris in the line and ensures proper infusion and yields a more accurate dosage of the medication. The solution is easily administered using a regulated flow and offers the merit of minimizing exposure of germs and organisms to the patient’s bloodstream. It is suitable for use in all health care settings and offers superior reliability to maximize efficacy.
BD 306546 PosiFlush IV Flush Solution Sodium Chloride, Preservative Free 0.9% Injection Prefilled Syringe 10 mL, 30/box
| Manufacturer # | 306546 |
| Brand | BD PosiFlush™ |
| Manufacturer | Becton Dickinson |
| Application | IV Flush Solution |
| Container Type | Prefilled Syringe |
| Dosage Form | Injection |
| Generic Drug Name | Sodium Chloride, Preservative Free |
| Storage Requirements | USP Controlled Room Temperature |
| Strength | 0.9% |
| Type | Intravenous |
| Volume | 10 mL |
Shipping Countries: United States (US)
Shipping States: Rhode Island (United States (US)), Nebraska (United States (US)), Nevada (United States (US)), New Hampshire (United States (US)), New Jersey (United States (US)), New Mexico (United States (US)), New York (United States (US)), North Carolina (United States (US)), North Dakota (United States (US)), Ohio (United States (US)), Oklahoma (United States (US)), Oregon (United States (US)), Pennsylvania (United States (US)), Montana (United States (US)), South Carolina (United States (US)), South Dakota (United States (US)), Tennessee (United States (US)), Texas (United States (US)), Utah (United States (US)), Vermont (United States (US)), Virginia (United States (US)), Washington (United States (US)), West Virginia (United States (US)), Wisconsin (United States (US)), Wyoming (United States (US)), Indiana (United States (US)), Alabama (United States (US)), Arkansas (United States (US)), California (United States (US)), Colorado (United States (US)), Connecticut (United States (US)), Delaware (United States (US)), District Of Columbia (United States (US)), Florida (United States (US)), Georgia (United States (US)), Idaho (United States (US)), Illinois (United States (US)), Arizona (United States (US)), Kansas (United States (US)), Iowa (United States (US)), Kentucky (United States (US)), Louisiana (United States (US)), Maine (United States (US)), Maryland (United States (US)), Michigan (United States (US)), Massachusetts (United States (US)), Minnesota (United States (US)), Mississippi (United States (US)), Missouri (United States (US))
Ready to ship in 1-2 business days
Shipping Policy
Products will be shipped per the method chosen at checkout from New York within 1 to 5 business days from the date that the order was placed (subject to stock & availability).
If a product is needed by a specific date, please call to ensure the product is in stock. Toll Free 1-888-687-4334.
Rx Items usually take 24 hours to complete and ship.
Refrigerated products will be shipped via Fedex overnight shipping Monday through Thursday. The customer is responsible to coordinate the tracking information and delivery time so these items will not be left out in the weather ailments.
Refund Policy
Returns are NOT accepted for prescription / RX items due to federal regulations on temperature control standards.
Please include receipt/order number and reason for the return.
Products must be unopened due to regulations from the department of health. All medical products are to be sold in the original packaging and cannot be repackaged.
All products to be returned must be returned within 30 days from the invoice date, 100% complete, in resalable condition, and must include original packing material, manuals, blank warranty cards and other accessories provided by the manufacturer.
All returns authorized by us are subject to a restocking fee of 25%, and must be completed within 30 days of the date of invoice/Sales receipt.
















Reviews
There are no reviews yet.