Hospira
-
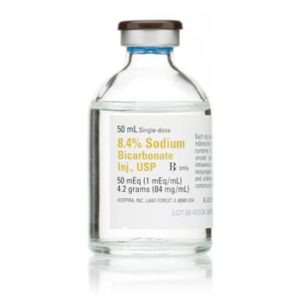
Sodium Bicarbonate 024916906832
Sterile sodium bicarbonate 8.4% injection USP for clinical use. Preservative-free solution for metabolic acidosis correction and pH adjustment in medical settings.
Price range: $18.99 through $425.00 -
Lidocaine 2%, 20mg/mL 50 mL 031386446000
Manufacturer: Hospira
Brand Generic Equivalent to Xylocaine®
Application Local Anesthetic
Strength: 2%, 20mg/mL
Generic Lidocaine HCl
Multiple Dose Vial
Storage Requirements USP Controlled Room Temperature
Type Injection, Inflitration and Nerve Block
Volume 50 mL
Latex Informationindicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system$6.99 -
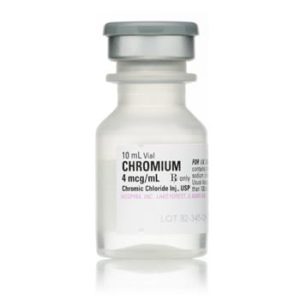
Chromium (Chromic Chloride Injection, USP) 028745525000
NDC Number 0040909301
Manufacturer: Hospira
Strength: 4mcg/mL
Generic Equivalent to Marcaine®, Sensorcaine®
Single Dose Vial
Generic Name Chromic Chloride
Storage Requirements USP Controlled Room Temperature
Type Intravenous
Volume 10 mL
Latex Information indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system.$30.00 -
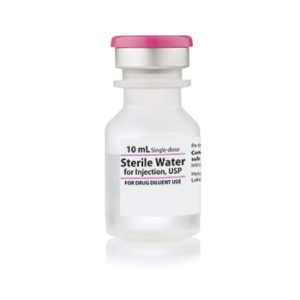
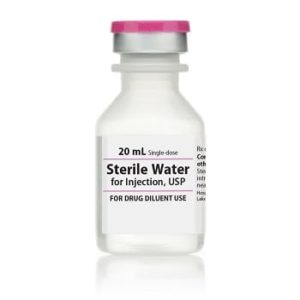
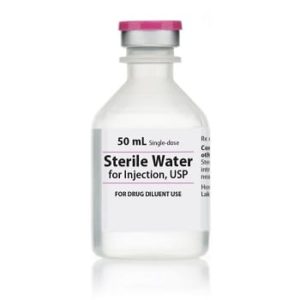
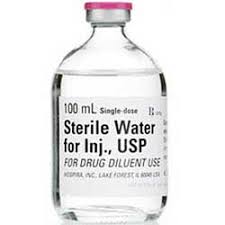
Sterile Water for Injection
Sterile Water for Injection, Preservative Free USP
Application: Diluent
Single Dose Fliptop Vial
Non-DEHP
Preservative Free
Latex Free: Latex Information: Natural rubber latex has not been used in the manufacture of this device or drug container closure system
Manufacturer: HospiraPrice range: $15.99 through $326.99 -
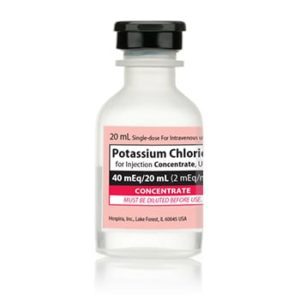
Potassium Chloride Concentrate 030827238000
NDC Number 00409-6653-05 / 00409665305
Manufacturer: Hospira
Application Replacement Preparation
Strength: 2 mEq/mL
Generic Equivalent to Marcaine®, Sensorcaine®
Single Dose Vial
Storage Requirements USP Controlled Room Temperature
Type Intravenous
Volume 20 mL
Latex Information indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system$9.50 -
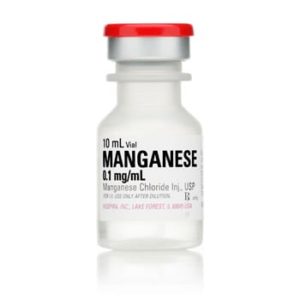
Manganese Chloride Injection USP, Preservative Free 0.1 mg / mL 10 mL 03150675000
NDC Number 00409-4091-01 / 00409409101
Manufacturer: Hospira
Application Trace Element
Strength: 0.1mf/mL
Generic Equivalent to Marcaine®, Sensorcaine®
Single Dose Vial
Storage Requirements USP Controlled Room Temperature
Type Intravenous
Volume 10 mL
Latex Information indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system$46.99 -
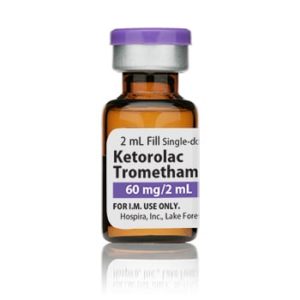
Ketorolac Tromethamine Injection
Manufacturer: Hospira
Application Nonsteroidal Anti-inflammatory Agent
Strength: 30mg/mL
Generic Ketorolac Tromethamine, Preservative Free
Single Dose Vial
Storage Requirements USP Controlled Room Temperature
Latex Information indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure systemPrice range: $49.99 through $54.99 -
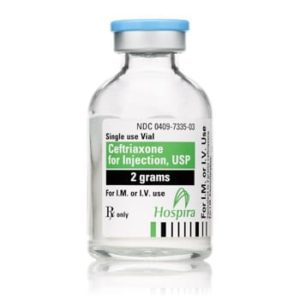
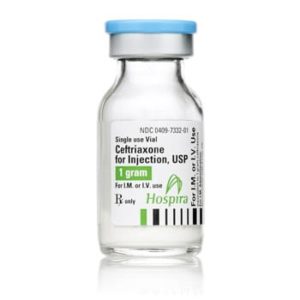
Ceftriaxone for Injection
Ceftriaxone for Injection, USP
Brand Name Equivalent: Rocephin®
Manufacturer Hospira/Pfizer
15mL Single Dose Vial“Latex Information” indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system.
“Preservative Information” indicates that this product does not contain ingredients identified as a preservative, as defined by the USP.
Price range: $21.00 through $53.00 -
Bacteriostatic Water for Injection Intramuscular 029450054500 | 30mL Vials, 25-Pack
NDC Number 00409-3977-03
NDC 00409397703
Manufacturer: Hospira
Application Diluent
Container Type Multiple Dose Vial
Generic Drug Name Bacteriostatic Water for Injection
Storage Requirements USP Controlled Room Temperature
Type Intramuscular, Intravenous, or Subcutaneous$289.99
Hospira was an American global pharmaceutical and medical device company with headquarters in Lake Forest, Illinois. It had approximately 19,000 employees.