- Medspa Essentials
- Aestheticians
- Apparel
- Clinic Furniture
- Devices
- Pharmaceutical & Medical Supplies
- Safety Supplies
- Training & Services
- Vendors
- Acara Partners
- Aesthetic Next
- Aesthetic Record
- American Med Spa Association
- Bonsai
- CEDR HR Solutions
- Colorescience
- CosmoGlo
- Derma Made
- Docovia
- Eden Skin And Body
- FACE Med Store
- KA Ring Designs
- LIFTIE Aspirator
- Massage Tools
- Merit Pharmaceutical
- National Medical Directors
- Post Love Skincare
- Rana Kennelley
- Source One Beauty
- SyringeRack
- The Aesthetic Immersion
- Velez by Vesna
- We Treat
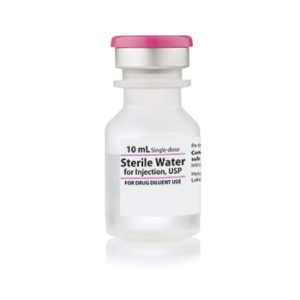
Bacteriostatic Water for Injection Intramuscular 029450054500 | 30mL Vials, 25-Pack
$289.99
NDC Number 00409-3977-03
NDC 00409397703
Manufacturer: Hospira
Application Diluent
Container Type Multiple Dose Vial
Generic Drug Name Bacteriostatic Water for Injection
Storage Requirements USP Controlled Room Temperature
Type Intramuscular, Intravenous, or Subcutaneous
SKU
00409397703
Categories Bacteriostatic Sodium Chloride, Medspa Essentials, Pharmacy, Prescription (Rx) Drugs & Supplies, sale
Tags 30 mL, Bacteriostatic Water, injection, Intramuscular, Intravenous, Latex, Merit Pharmaceutical, Multiple Dose Vial, Pack 25 Vials, Subcutaneous Injection, Water
Brand: Hospira
Hospira Bacteriostatic Water for Injection 30mL Vials, 25-Pack
Hospira’s Bacteriostatic Water for Injection (NDC 00409-3977-03) is a sterile, non-pyrogenic solution formulated for the safe dilution and reconstitution of injectable medications. Each 30mL multiple-dose vial contains USP-grade water preserved with 0.9% benzyl alcohol, which inhibits microbial growth and allows for repeated needle entries without compromising sterility. This preservative makes it ideal for multi-dose protocols in clinical environments.
Designed for intramuscular, intravenous, or subcutaneous administration when mixed with compatible drugs, this product is widely used in medspas, hospitals, compounding pharmacies, and research labs. It supports the preparation of peptides, hormone therapies, biologics, and other injectable treatments that require precise dilution protocols. The solution is not intended for direct injection and must be used only as a vehicle for approved medications.
Hospira’s packaging ensures safety and compliance. The container closure system is latex-free, reducing the risk of allergic reactions and meeting the needs of sensitive patients. The vials are manufactured to meet USP standards for controlled room temperature storage, ensuring product integrity throughout its shelf life. Each vial should be discarded 28 days after first entry or sooner if contamination is suspected.
Healthcare professionals trust Hospira for consistent quality, reliable supply, and pharmaceutical-grade sterility. Whether you’re managing a high-volume medspa or a clinical research facility, this 25-pack of 30mL vials offers convenience, safety, and performance you can count on. The product is Rx-only and should be handled using aseptic technique to maintain sterility.
Additional information
| Weight | 3 kg |
|---|---|
| Dimensions | 6.75 × 6.75 × 3 cm |
-
$9.99 – $272.00Price range: $9.99 through $272.00Sold By: Merit Pharmaceutical
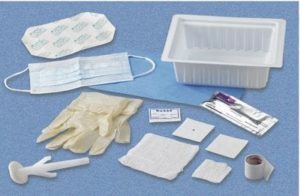
Central Line Tray With ChloraPrep One-Step Applicator 078121197500
$9.99 – $272.00Price range: $9.99 through $272.00 Select options -
$1.00 – $50.00Price range: $1.00 through $50.00Sold By: Merit Pharmaceutical
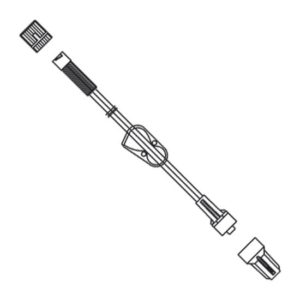
Extension Set, Minibore, 7”, Meritset 036001170000
$1.00 – $50.00Price range: $1.00 through $50.00 Select options -
$66.50
-
$1.99 – $62.50Price range: $1.99 through $62.50Sold By: Merit Pharmaceutical
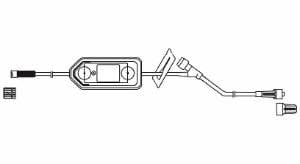
Extension Set, Regular Bore, 7”, Meriset 088713723000
$1.99 – $62.50Price range: $1.99 through $62.50 Select options
Hospira Bacteriostatic Water for Injection 30mL Vials, 25-Pack
Hospira’s Bacteriostatic Water for Injection (NDC 00409-3977-03) is a sterile, non-pyrogenic solution formulated for the safe dilution and reconstitution of injectable medications. Each 30mL multiple-dose vial contains USP-grade water preserved with 0.9% benzyl alcohol, which inhibits microbial growth and allows for repeated needle entries without compromising sterility. This preservative makes it ideal for multi-dose protocols in clinical environments.
Designed for intramuscular, intravenous, or subcutaneous administration when mixed with compatible drugs, this product is widely used in medspas, hospitals, compounding pharmacies, and research labs. It supports the preparation of peptides, hormone therapies, biologics, and other injectable treatments that require precise dilution protocols. The solution is not intended for direct injection and must be used only as a vehicle for approved medications.
Hospira’s packaging ensures safety and compliance. The container closure system is latex-free, reducing the risk of allergic reactions and meeting the needs of sensitive patients. The vials are manufactured to meet USP standards for controlled room temperature storage, ensuring product integrity throughout its shelf life. Each vial should be discarded 28 days after first entry or sooner if contamination is suspected.
Healthcare professionals trust Hospira for consistent quality, reliable supply, and pharmaceutical-grade sterility. Whether you’re managing a high-volume medspa or a clinical research facility, this 25-pack of 30mL vials offers convenience, safety, and performance you can count on. The product is Rx-only and should be handled using aseptic technique to maintain sterility.
Additional information
| Weight | 3 kg |
|---|---|
| Dimensions | 6.75 × 6.75 × 3 cm |
-
$9.99 – $272.00Price range: $9.99 through $272.00Sold By: Merit Pharmaceutical
Central Line Tray With ChloraPrep One-Step Applicator 078121197500
$9.99 – $272.00Price range: $9.99 through $272.00 Select options -
$1.00 – $50.00Price range: $1.00 through $50.00Sold By: Merit Pharmaceutical
Extension Set, Minibore, 7”, Meritset 036001170000
$1.00 – $50.00Price range: $1.00 through $50.00 Select options -
$66.50
-
$1.99 – $62.50Price range: $1.99 through $62.50Sold By: Merit Pharmaceutical
Extension Set, Regular Bore, 7”, Meriset 088713723000
$1.99 – $62.50Price range: $1.99 through $62.50 Select options
Hospira Bacteriostatic Water for Injection 30mL Vials, 25-Pack
Hospira’s Bacteriostatic Water for Injection (NDC 00409-3977-03) is a sterile, non-pyrogenic solution formulated for the safe dilution and reconstitution of injectable medications. Each 30mL multiple-dose vial contains USP-grade water preserved with 0.9% benzyl alcohol, which inhibits microbial growth and allows for repeated needle entries without compromising sterility. This preservative makes it ideal for multi-dose protocols in clinical environments.
Designed for intramuscular, intravenous, or subcutaneous administration when mixed with compatible drugs, this product is widely used in medspas, hospitals, compounding pharmacies, and research labs. It supports the preparation of peptides, hormone therapies, biologics, and other injectable treatments that require precise dilution protocols. The solution is not intended for direct injection and must be used only as a vehicle for approved medications.
Hospira’s packaging ensures safety and compliance. The container closure system is latex-free, reducing the risk of allergic reactions and meeting the needs of sensitive patients. The vials are manufactured to meet USP standards for controlled room temperature storage, ensuring product integrity throughout its shelf life. Each vial should be discarded 28 days after first entry or sooner if contamination is suspected.
Healthcare professionals trust Hospira for consistent quality, reliable supply, and pharmaceutical-grade sterility. Whether you’re managing a high-volume medspa or a clinical research facility, this 25-pack of 30mL vials offers convenience, safety, and performance you can count on. The product is Rx-only and should be handled using aseptic technique to maintain sterility.
Additional information
| Weight | 3 kg |
|---|---|
| Dimensions | 6.75 × 6.75 × 3 cm |
-
$9.99 – $272.00Price range: $9.99 through $272.00Sold By: Merit Pharmaceutical
Central Line Tray With ChloraPrep One-Step Applicator 078121197500
$9.99 – $272.00Price range: $9.99 through $272.00 Select options -
$1.00 – $50.00Price range: $1.00 through $50.00Sold By: Merit Pharmaceutical
Extension Set, Minibore, 7”, Meritset 036001170000
$1.00 – $50.00Price range: $1.00 through $50.00 Select options -
$66.50
-
$1.99 – $62.50Price range: $1.99 through $62.50Sold By: Merit Pharmaceutical
Extension Set, Regular Bore, 7”, Meriset 088713723000
$1.99 – $62.50Price range: $1.99 through $62.50 Select options
Hospira Bacteriostatic Water for Injection 30mL Vials, 25-Pack
Hospira’s Bacteriostatic Water for Injection (NDC 00409-3977-03) is a sterile, non-pyrogenic solution formulated for the safe dilution and reconstitution of injectable medications. Each 30mL multiple-dose vial contains USP-grade water preserved with 0.9% benzyl alcohol, which inhibits microbial growth and allows for repeated needle entries without compromising sterility. This preservative makes it ideal for multi-dose protocols in clinical environments.
Designed for intramuscular, intravenous, or subcutaneous administration when mixed with compatible drugs, this product is widely used in medspas, hospitals, compounding pharmacies, and research labs. It supports the preparation of peptides, hormone therapies, biologics, and other injectable treatments that require precise dilution protocols. The solution is not intended for direct injection and must be used only as a vehicle for approved medications.
Hospira’s packaging ensures safety and compliance. The container closure system is latex-free, reducing the risk of allergic reactions and meeting the needs of sensitive patients. The vials are manufactured to meet USP standards for controlled room temperature storage, ensuring product integrity throughout its shelf life. Each vial should be discarded 28 days after first entry or sooner if contamination is suspected.
Healthcare professionals trust Hospira for consistent quality, reliable supply, and pharmaceutical-grade sterility. Whether you’re managing a high-volume medspa or a clinical research facility, this 25-pack of 30mL vials offers convenience, safety, and performance you can count on. The product is Rx-only and should be handled using aseptic technique to maintain sterility.
Additional information
| Weight | 3 kg |
|---|---|
| Dimensions | 6.75 × 6.75 × 3 cm |
-
$9.99 – $272.00Price range: $9.99 through $272.00Sold By: Merit Pharmaceutical
Central Line Tray With ChloraPrep One-Step Applicator 078121197500
$9.99 – $272.00Price range: $9.99 through $272.00 Select options -
$1.00 – $50.00Price range: $1.00 through $50.00Sold By: Merit Pharmaceutical
Extension Set, Minibore, 7”, Meritset 036001170000
$1.00 – $50.00Price range: $1.00 through $50.00 Select options -
$66.50
-
$1.99 – $62.50Price range: $1.99 through $62.50Sold By: Merit Pharmaceutical
Extension Set, Regular Bore, 7”, Meriset 088713723000
$1.99 – $62.50Price range: $1.99 through $62.50 Select options
Hospira Bacteriostatic Water for Injection 30mL Vials, 25-Pack
Hospira’s Bacteriostatic Water for Injection (NDC 00409-3977-03) is a sterile, non-pyrogenic solution formulated for the safe dilution and reconstitution of injectable medications. Each 30mL multiple-dose vial contains USP-grade water preserved with 0.9% benzyl alcohol, which inhibits microbial growth and allows for repeated needle entries without compromising sterility. This preservative makes it ideal for multi-dose protocols in clinical environments.
Designed for intramuscular, intravenous, or subcutaneous administration when mixed with compatible drugs, this product is widely used in medspas, hospitals, compounding pharmacies, and research labs. It supports the preparation of peptides, hormone therapies, biologics, and other injectable treatments that require precise dilution protocols. The solution is not intended for direct injection and must be used only as a vehicle for approved medications.
Hospira’s packaging ensures safety and compliance. The container closure system is latex-free, reducing the risk of allergic reactions and meeting the needs of sensitive patients. The vials are manufactured to meet USP standards for controlled room temperature storage, ensuring product integrity throughout its shelf life. Each vial should be discarded 28 days after first entry or sooner if contamination is suspected.
Healthcare professionals trust Hospira for consistent quality, reliable supply, and pharmaceutical-grade sterility. Whether you’re managing a high-volume medspa or a clinical research facility, this 25-pack of 30mL vials offers convenience, safety, and performance you can count on. The product is Rx-only and should be handled using aseptic technique to maintain sterility.
Additional information
| Weight | 3 kg |
|---|---|
| Dimensions | 6.75 × 6.75 × 3 cm |
-
$9.99 – $272.00Price range: $9.99 through $272.00Sold By: Merit Pharmaceutical
Central Line Tray With ChloraPrep One-Step Applicator 078121197500
$9.99 – $272.00Price range: $9.99 through $272.00 Select options -
$1.00 – $50.00Price range: $1.00 through $50.00Sold By: Merit Pharmaceutical
Extension Set, Minibore, 7”, Meritset 036001170000
$1.00 – $50.00Price range: $1.00 through $50.00 Select options -
$66.50
-
$1.99 – $62.50Price range: $1.99 through $62.50Sold By: Merit Pharmaceutical
Extension Set, Regular Bore, 7”, Meriset 088713723000
$1.99 – $62.50Price range: $1.99 through $62.50 Select options
Hospira Bacteriostatic Water for Injection 30mL Vials, 25-Pack
Hospira’s Bacteriostatic Water for Injection (NDC 00409-3977-03) is a sterile, non-pyrogenic solution formulated for the safe dilution and reconstitution of injectable medications. Each 30mL multiple-dose vial contains USP-grade water preserved with 0.9% benzyl alcohol, which inhibits microbial growth and allows for repeated needle entries without compromising sterility. This preservative makes it ideal for multi-dose protocols in clinical environments.
Designed for intramuscular, intravenous, or subcutaneous administration when mixed with compatible drugs, this product is widely used in medspas, hospitals, compounding pharmacies, and research labs. It supports the preparation of peptides, hormone therapies, biologics, and other injectable treatments that require precise dilution protocols. The solution is not intended for direct injection and must be used only as a vehicle for approved medications.
Hospira’s packaging ensures safety and compliance. The container closure system is latex-free, reducing the risk of allergic reactions and meeting the needs of sensitive patients. The vials are manufactured to meet USP standards for controlled room temperature storage, ensuring product integrity throughout its shelf life. Each vial should be discarded 28 days after first entry or sooner if contamination is suspected.
Healthcare professionals trust Hospira for consistent quality, reliable supply, and pharmaceutical-grade sterility. Whether you’re managing a high-volume medspa or a clinical research facility, this 25-pack of 30mL vials offers convenience, safety, and performance you can count on. The product is Rx-only and should be handled using aseptic technique to maintain sterility.
Additional information
| Weight | 3 kg |
|---|---|
| Dimensions | 6.75 × 6.75 × 3 cm |
-
$9.99 – $272.00Price range: $9.99 through $272.00Sold By: Merit Pharmaceutical
Central Line Tray With ChloraPrep One-Step Applicator 078121197500
$9.99 – $272.00Price range: $9.99 through $272.00 Select options -
$1.00 – $50.00Price range: $1.00 through $50.00Sold By: Merit Pharmaceutical
Extension Set, Minibore, 7”, Meritset 036001170000
$1.00 – $50.00Price range: $1.00 through $50.00 Select options -
$66.50
-
$1.99 – $62.50Price range: $1.99 through $62.50Sold By: Merit Pharmaceutical
Extension Set, Regular Bore, 7”, Meriset 088713723000
$1.99 – $62.50Price range: $1.99 through $62.50 Select options
Hospira Bacteriostatic Water for Injection 30mL Vials, 25-Pack
Hospira’s Bacteriostatic Water for Injection (NDC 00409-3977-03) is a sterile, non-pyrogenic solution formulated for the safe dilution and reconstitution of injectable medications. Each 30mL multiple-dose vial contains USP-grade water preserved with 0.9% benzyl alcohol, which inhibits microbial growth and allows for repeated needle entries without compromising sterility. This preservative makes it ideal for multi-dose protocols in clinical environments.
Designed for intramuscular, intravenous, or subcutaneous administration when mixed with compatible drugs, this product is widely used in medspas, hospitals, compounding pharmacies, and research labs. It supports the preparation of peptides, hormone therapies, biologics, and other injectable treatments that require precise dilution protocols. The solution is not intended for direct injection and must be used only as a vehicle for approved medications.
Hospira’s packaging ensures safety and compliance. The container closure system is latex-free, reducing the risk of allergic reactions and meeting the needs of sensitive patients. The vials are manufactured to meet USP standards for controlled room temperature storage, ensuring product integrity throughout its shelf life. Each vial should be discarded 28 days after first entry or sooner if contamination is suspected.
Healthcare professionals trust Hospira for consistent quality, reliable supply, and pharmaceutical-grade sterility. Whether you’re managing a high-volume medspa or a clinical research facility, this 25-pack of 30mL vials offers convenience, safety, and performance you can count on. The product is Rx-only and should be handled using aseptic technique to maintain sterility.
Additional information
| Weight | 3 kg |
|---|---|
| Dimensions | 6.75 × 6.75 × 3 cm |
-
$9.99 – $272.00Price range: $9.99 through $272.00Sold By: Merit Pharmaceutical
Central Line Tray With ChloraPrep One-Step Applicator 078121197500
$9.99 – $272.00Price range: $9.99 through $272.00 Select options -
$1.00 – $50.00Price range: $1.00 through $50.00Sold By: Merit Pharmaceutical
Extension Set, Minibore, 7”, Meritset 036001170000
$1.00 – $50.00Price range: $1.00 through $50.00 Select options -
$66.50
-
$1.99 – $62.50Price range: $1.99 through $62.50Sold By: Merit Pharmaceutical
Extension Set, Regular Bore, 7”, Meriset 088713723000
$1.99 – $62.50Price range: $1.99 through $62.50 Select options
Best selling
-
Easy Touch Insulin
$15.99 -
Non-Woven Sponges
$3.50 -
IV Start Kit Latex Free
$77.00
-
-
Sharps Container
$5.25
-
Related Products
Related Products
-
$15.99 – $326.99Price range: $15.99 through $326.99Sold By: Merit Pharmaceutical
Sterile Water for Injection
$15.99 – $326.99Price range: $15.99 through $326.99 Select options -
$21.00 – $53.00Price range: $21.00 through $53.00Sold By: Merit Pharmaceutical
Ceftriaxone for Injection
$21.00 – $53.00Price range: $21.00 through $53.00 Select options
-
$15.99 – $326.99Price range: $15.99 through $326.99Sold By: Merit Pharmaceutical
Sterile Water for Injection
$15.99 – $326.99Price range: $15.99 through $326.99 Select options -
$21.00 – $53.00Price range: $21.00 through $53.00Sold By: Merit Pharmaceutical
Ceftriaxone for Injection
$21.00 – $53.00Price range: $21.00 through $53.00 Select options
-
$650.00
$850.00Sold By: Rana KennelleyTwo Accredited Neuromodulator On-Line Training Courses
$650.00$850.00Add to cart -
$790.00
$990.00Sold By: Rana KennelleyTwo Accredited Facial Filler On-Line Training Courses
$790.00$990.00Add to cart -
$91.00
$113.99 -
Manual Bundle
Sold By: Acara Partners$1,250.00$1,750.00 -
Collins Cameo Reception Desk with 10 Color Options ($1 Shipping) Made in USA
Sold By: Source One Beauty$1,009.00$1,062.00Sold By: Source One BeautyCollins Cameo Reception Desk with 10 Color Options ($1 Shipping) Made in USA
$1,009.00$1,062.00Select options -
Collins Reve Concierge Desk with 10 Color Options ($1 Shipping) Made in USA
Sold By: Source One Beauty$1,031.50$1,086.00Sold By: Source One BeautyCollins Reve Concierge Desk with 10 Color Options ($1 Shipping) Made in USA
$1,031.50$1,086.00Select options -
Collins Reve Retail Display with 10 Color Options ($1 Shipping) Made in USA
Sold By: Source One Beauty$838.00$882.00 -
Collins Amati Retail Display with 10 Color Options ($1 Shipping) Made in USA
Sold By: Source One Beauty$792.50$834.00 -
$2,462.50
$2,592.00
-
$650.00
$850.00Sold By: Rana KennelleyTwo Accredited Neuromodulator On-Line Training Courses
$650.00$850.00Add to cart -
$790.00
$990.00Sold By: Rana KennelleyTwo Accredited Facial Filler On-Line Training Courses
$790.00$990.00Add to cart -
$91.00
$113.99 -
Manual Bundle
Sold By: Acara Partners$1,250.00$1,750.00 -
Collins Cameo Reception Desk with 10 Color Options ($1 Shipping) Made in USA
Sold By: Source One Beauty$1,009.00$1,062.00Sold By: Source One BeautyCollins Cameo Reception Desk with 10 Color Options ($1 Shipping) Made in USA
$1,009.00$1,062.00Select options -
Collins Reve Concierge Desk with 10 Color Options ($1 Shipping) Made in USA
Sold By: Source One Beauty$1,031.50$1,086.00Sold By: Source One BeautyCollins Reve Concierge Desk with 10 Color Options ($1 Shipping) Made in USA
$1,031.50$1,086.00Select options -
Collins Reve Retail Display with 10 Color Options ($1 Shipping) Made in USA
Sold By: Source One Beauty$838.00$882.00 -
Collins Amati Retail Display with 10 Color Options ($1 Shipping) Made in USA
Sold By: Source One Beauty$792.50$834.00 -
$2,462.50
$2,592.00