Latex
-
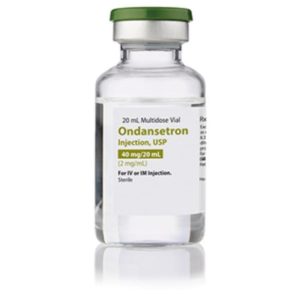
Ondansetron HCL, MDV 2mg/mL 20mL
NDC Number 16729-0298-05 / 16729029805
Manufacturer: Accord Healthcare
Application Prevention of Nausea and Vomiting
Strength: 2mg/mL
Multiple Dose Vial
Storage Requirements USP Controlled Room Temperature
Type Intramuscular, Intravenous, or Subcutaneous
Volume 20 mL
Latex Information indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system$9.99 -
Transform Nitrile Powder-Free Examination Gloves 200/Pack
We promise that Transform® will “re-shape” the way you feel about Nitrile gloves. Our formula? Long-lasting comfort, durability and decreased hand fatigue. Transform® offers improved barrier protection while mimicking the slim fit and comfort of a latex glove.
Contains: 200/gloves per box
$14.95 -
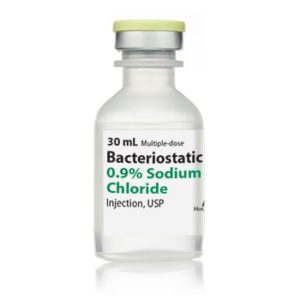
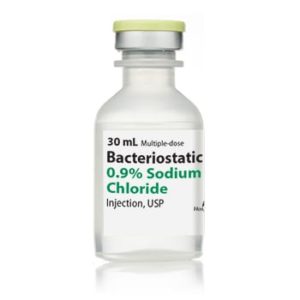
Bacteriostatic Water for Injection Intramuscular
NDC Number 00409-3977-03
NDC 00409397703
Manufacturer: Hospira
Application Diluent
Container Type Multiple Dose Vial
Generic Drug Name Bacteriostatic Water for Injection
Storage Requirements USP Controlled Room Temperature
Type Intramuscular, Intravenous, or SubcutaneousVolume 30 mL, Pack of 25 VIALS
Latex Information indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system.
“Preservative Information” indicates that this product does not contain ingredients identified as a preservative, as defined by the USP$66.99 -
Dexamethasone Sodium Phosphate
Dexamethasone Sodium Phosphate 4 mg / mL Intramuscular or Intravenous Injection Multiple Dose Vial 30 mL
NDC Number 6745742130 / 67457-421-30
Manufacturer: Mylan Institutional
Application Corticosteroid
Strength: 4mg/mL
Multiple Dose Vial
Generic Name Dexamethasone Sodium Phosphate
Storage Requirements USP Controlled Room Temperature
Type Intramuscular or Intravenous / Injection
Volume 30 mL
Latex Information indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system$23.70 -
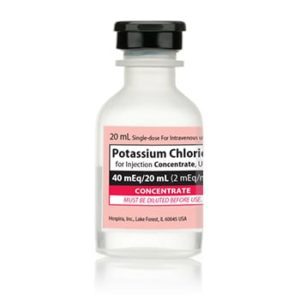
Potassium Chloride Concentrate
NDC Number 00409-6653-05 / 00409665305
Manufacturer: Hospira
Application Replacement Preparation
Strength: 2 mEq/mL
Generic Equivalent to Marcaine®, Sensorcaine®
Single Dose Vial
Storage Requirements USP Controlled Room Temperature
Type Intravenous
Volume 20 mL
Latex Information indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system$9.50 -
0.2 Micron SUPOR Aspiration / Injection Disc Filter
Braun # 415002, 0.2 Micron Supor Aspiration/ Injection Disc Filter
Proximal and distal Luer lock connections designed for bacteria retentive filtration of medication
Fluid retention: 0.3 mL
Color: Green
Product Identification
Product Code PF2000
NDC Number 08021-4150-02
Reference Number 415002
Safety Data
Latex Information Components Do Not Contain Natural Rubber$2.35 – $116.99