- Medspa Essentials
- Aestheticians
- Apparel
- Clinic Furniture
- Devices
- Pharmaceutical & Medical Supplies
- Safety Supplies
- Training & Services
- Vendors
- Acara Partners
- Aesthetic Next
- Aesthetic Record
- American Med Spa Association
- Bonsai
- CEDR HR Solutions
- Colorescience
- CosmoGlo
- Derma Made
- Docovia
- Eden Skin And Body
- FACE Med Store
- KA Ring Designs
- LIFTIE Aspirator
- Massage Tools
- Merit Pharmaceutical
- National Medical Directors
- Post Love Skincare
- Rana Kennelley
- Renee John Uniforms
- Source One Beauty
- SyringeRack
- The Aesthetic Immersion
- Velez by Vesna
- We Treat
Flash sale
Fresh deal everyday. Get it before time runs out
-
$50.99
$64.99Disposable Fabric Pillow Covers – 21″ x 23″ – 100PK (5 x 20PK)
Sold By: Source One Beauty -
$2,449.00
$3,099.00Spa Numa NEW MEDICI Heavy Duty Medical Grade Pedestal 4-Motor Treatment Chair
Sold By: Source One Beauty
Product Categories
- All Categories
- Pharmacy
- Over The Counter (OTC) Drugs & Supplies
- Prescription (Rx) Drugs & Supplies
- Supplements & Nutrition
Show MoreShow Less
Filter by price
Color
Size
Edit Content
Product Categories
- All Categories
- Pharmacy
- Over The Counter (OTC) Drugs & Supplies
- Prescription (Rx) Drugs & Supplies
- Supplements & Nutrition
Show MoreShow Less
Filter by price
Color
Size
104Products found
Filter
-
Metformin ER Tablets 500mg, 500 / Bottle NDC 024345966671
Sold By: Merit PharmaceuticalNDC 51224-0007-60
Manufacturer. Tagi
Package Size 500
Strength 500MG
Color WhiteNon-Returnable
$14.99 -
Lidocaine 2%, 20mg/mL 50 mL 031386446000
Sold By: Merit PharmaceuticalManufacturer: Hospira
Brand Generic Equivalent to Xylocaine®
Application Local Anesthetic
Strength: 2%, 20mg/mL
Generic Lidocaine HCl
Multiple Dose Vial
Storage Requirements USP Controlled Room Temperature
Type Injection, Inflitration and Nerve Block
Volume 50 mL
Latex Informationindicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system$6.99 -
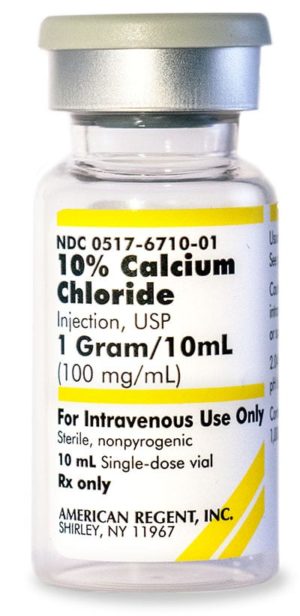
10% Calcium Chloride Injection, USP 028867198000
Sold By: Merit PharmaceuticalNDC Number 0517-6710-10
NDC Number 00517671010
Manufacturer: American Regent
Application Replacement Preparation
Strength: 100mg/mL
Single Dose Vial
Storage Requirements USP Controlled Room Temperature
Type Intravenous
Volume 10 mL$33.99 – $339.90Price range: $33.99 through $339.90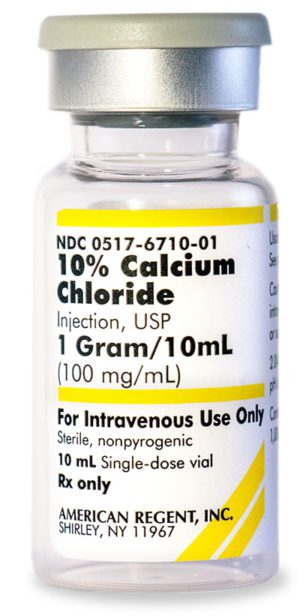 Sold By: Merit Pharmaceutical
Sold By: Merit Pharmaceutical10% Calcium Chloride Injection, USP 028867198000
$33.99 – $339.90Price range: $33.99 through $339.90 Select options -
Calcium Chloride, Preservative Free 028930786000
Sold By: Merit PharmaceuticalNDC Number Varies
Manufacturer: Various
Application Replacement Preparation
Strength: 10%, 100mg/mL
Generic Equivalent to Marcaine®, Sensorcaine®
Generic Name Calcium Chloride, Preservative Free
Storage Requirements USP Controlled Room Temperature
Type Intravenous
Volume 10 mL
Latex Information: Latex Free.$20.99 -
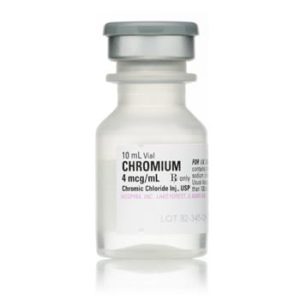
Chromium (Chromic Chloride Injection, USP) 028745525000
Sold By: Merit PharmaceuticalNDC Number 0040909301
Manufacturer: Hospira
Strength: 4mcg/mL
Generic Equivalent to Marcaine®, Sensorcaine®
Single Dose Vial
Generic Name Chromic Chloride
Storage Requirements USP Controlled Room Temperature
Type Intravenous
Volume 10 mL
Latex Information indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system.$30.00 -
CYANOCOBALAMIN PACKAGE
Sold By: Merit PharmaceuticalSUPER B-12 PACKAGE WITH CYANOCOBALMIN 30ML (COBAL 1000) INJECTION, USP, MDV, 1000MCG, NDC # 67457-400-05
Package Items Include:
5 x 30mL Cyanocobalamin MDV
Choice of One Box:
_____ Insulin Syringes 1cc 30G x 5/16, Bx/100
_____ Insulin Syringes 1/3cc 31 x 5/16, Bx/100
_____3cc 23G x 1”, Bx/100 #26101
_____3cc 25G x 5/8, Bx/100 #26100
_____3cc 25G x 1”, Bx/100 #044327777
Included:
_____Alcohol Prep Pads, Medium, Bx/200
_____Sharps Container, Red, 2 Gallon
_____ Band-aids, Bx/100, 1” x 3” or Spots
$150.00 -
Sterile Water for Injection
Sold By: Merit PharmaceuticalSterile Water for Injection, Preservative Free USP
Application: Diluent
Single Dose Fliptop Vial
Non-DEHP
Preservative Free
Latex Free: Latex Information: Natural rubber latex has not been used in the manufacture of this device or drug container closure system
Manufacturer: Hospira$103.99 -
Solu-Medrol Methylprednisolone Sodium Succinate 40MG 032365935000
Sold By: Merit PharmaceuticalNDC Number 00009-00039-28 / 00009003928
Brand Solu-Medrol®
Manufacturer: Pfizer
Application Corticosteroid
Strength: 40mg/mL
Generic Equivalent to Methylprednisolone Sodium Succinate, Preservative Free
Single Dose Vial
Storage Requirements USP Controlled Room Temperature
Type Intramuscular, Intravenous, or Subcutaneous
Volume 1 mL$9.99 -
Solu-Medrol Methylprednisolone Sodium Succinate 032336805000
Sold By: Merit PharmaceuticalNDC Number 00009-0047-22 / 0009004722
Manufacturer Pfizer
Brand Solu-Medrol®
Application Corticosteroid
Strength: 125mg/2mL
Generic Equivalent to Marcaine®, Sensorcaine®
Single Dose Vial – Act-O-Vial
Storage Requirements USP Controlled Room Temperature
Volume 2 mL$14.99 -
Potassium Chloride Concentrate 030827238000
Sold By: Merit PharmaceuticalNDC Number 00409-6653-05 / 00409665305
Manufacturer: Hospira
Application Replacement Preparation
Strength: 2 mEq/mL
Generic Equivalent to Marcaine®, Sensorcaine®
Single Dose Vial
Storage Requirements USP Controlled Room Temperature
Type Intravenous
Volume 20 mL
Latex Information indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system$9.50 -
Ondansetron HCL, MDV 2mg/mL 20mL 032158586812
Sold By: Merit PharmaceuticalNDC Number 16729-0298-05 / 16729029805
Manufacturer: Accord Healthcare
Application Prevention of Nausea and Vomiting
Strength: 2mg/mL
Multiple Dose Vial
Storage Requirements USP Controlled Room Temperature
Type Intramuscular, Intravenous, or Subcutaneous
Volume 20 mL
Latex Information indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system$9.99 -
Solu-Cortef
Sold By: Merit PharmaceuticalManufacturer: Pfizer
Brand Solu-Cortef®
Generic Drug Name Hydrocortisone Sodium Succinate, Preservative Free
Application Anti-inflammatory Agent
Single Dose Vial
Storage Requirements USP Controlled Room Temperature
Type Intramuscular or Intravenous$30.99 – $113.99Price range: $30.99 through $113.99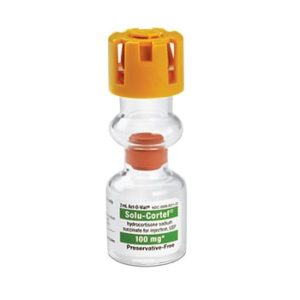

 Sold By: Merit Pharmaceutical
Sold By: Merit PharmaceuticalSolu-Cortef
$30.99 – $113.99Price range: $30.99 through $113.99 Select options -
Sildenafil Citrate 50 mg 024916848898
Sold By: Merit PharmaceuticalNDC # 27241-0068-03 / 27241006803
Manufacturer Ajanta Pharma Limited
Erectile Dysfunction Agent
Tablets
Generic Name: Sildenafil Citrate
30 Tablets Per Bottle
Non-Returnable
Note: Manufacturer May Vary$30.99 -
Manganese Chloride Injection USP, Preservative Free 0.1 mg / mL 10 mL 03150675000
Sold By: Merit PharmaceuticalNDC Number 00409-4091-01 / 00409409101
Manufacturer: Hospira
Application Trace Element
Strength: 0.1mf/mL
Generic Equivalent to Marcaine®, Sensorcaine®
Single Dose Vial
Storage Requirements USP Controlled Room Temperature
Type Intravenous
Volume 10 mL
Latex Information indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system$46.99 -
Ketorolac Tromethamine Injection
Sold By: Merit PharmaceuticalManufacturer: Hospira
Application Nonsteroidal Anti-inflammatory Agent
Strength: 30mg/mL
Generic Ketorolac Tromethamine, Preservative Free
Single Dose Vial
Storage Requirements USP Controlled Room Temperature
Latex Information indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system$49.99 – $54.99Price range: $49.99 through $54.99
 Sold By: Merit Pharmaceutical
Sold By: Merit PharmaceuticalKetorolac Tromethamine Injection
$49.99 – $54.99Price range: $49.99 through $54.99 Select options -
Kenalog
Sold By: Merit PharmaceuticalManufacturer: Bristol-Myers Squibb
Brand Kenalog®-40
Application Corticosteroid
Generic Equivalent to Marcaine®, Sensorcaine®
Multiple Dose Vial
Storage Requirements USP Controlled Room Temperature
Type Injectable / Injection / Intramuscular or Intra-Asticular$65.00 -
Dexamethasone Sodium Phosphate 029643576000
Sold By: Merit PharmaceuticalDexamethasone Sodium Phosphate 4 mg / mL Intramuscular or Intravenous Injection Multiple Dose Vial 30 mL
NDC Number 6745742130 / 67457-421-30
Manufacturer: Mylan Institutional
Application Corticosteroid
Strength: 4mg/mL
Multiple Dose Vial
Generic Name Dexamethasone Sodium Phosphate
Storage Requirements USP Controlled Room Temperature
Type Intramuscular or Intravenous / Injection
Volume 30 mL
Latex Information indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system$23.70 -
Ceftriaxone for Injection
Sold By: Merit PharmaceuticalCeftriaxone for Injection, USP
Brand Name Equivalent: Rocephin®
Manufacturer Hospira/Pfizer
15mL Single Dose Vial“Latex Information” indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system.
“Preservative Information” indicates that this product does not contain ingredients identified as a preservative, as defined by the USP.
$21.00 – $53.00Price range: $21.00 through $53.00
 Sold By: Merit Pharmaceutical
Sold By: Merit PharmaceuticalCeftriaxone for Injection
$21.00 – $53.00Price range: $21.00 through $53.00 Select options -
Bacteriostatic Water for Injection Intramuscular 029450054500 | 30mL Vials, 25-Pack
Sold By: Merit PharmaceuticalNDC Number 00409-3977-03
NDC 00409397703
Manufacturer: Hospira
Application Diluent
Container Type Multiple Dose Vial
Generic Drug Name Bacteriostatic Water for Injection
Storage Requirements USP Controlled Room Temperature
Type Intramuscular, Intravenous, or Subcutaneous$250.00 -
3M Durapore Silk Tape
Sold By: Merit PharmaceuticalBrand 3M™ Durapore™
Manufacturer 3M
Medical Silk Tape
Non-Sterile
Latex Free$19.99 -
3M Micropore Surgical Tape
Sold By: Merit PharmaceuticalBrand 3M™ Micropore™
Manufacturer 3M
White
Medical Paper Tape
Non-Sterile
Latex Free$8.99 – $12.99Price range: $8.99 through $12.99 Sold By: Merit Pharmaceutical
Sold By: Merit Pharmaceutical3M Micropore Surgical Tape
$8.99 – $12.99Price range: $8.99 through $12.99 Select options -
$2.99 – $21.99Price range: $2.99 through $21.99
 Sold By: Merit Pharmaceutical
Sold By: Merit PharmaceuticalTriple Antibiotic Antibiotic Ointment
$2.99 – $21.99Price range: $2.99 through $21.99 Select options -
Hydrogen Peroxide 3% 16OZ 025760401000
Sold By: Merit PharmaceuticalHydrogen Peroxide 3%
16 fl oz (1 pt) (473 mL)
Topical Solution USP
First Aid antiseptic and cleaning agent.
For treatment of minor cuts, burns and abrasions.
Packaged: EACH. To receive a case, please order 12.$0.99 -
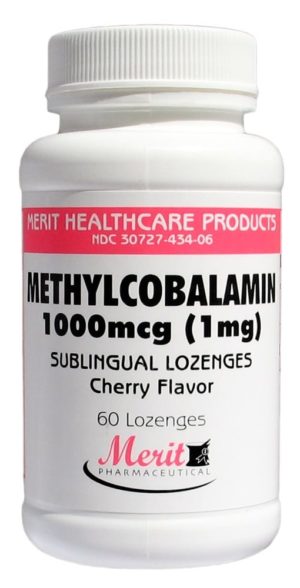
Methylcobalamin 1000mcg / 1mg Cherry Flavor, 60 Sublingual Lozenges 006313560000
Sold By: Merit PharmaceuticalEach 1 Lozenge:
Methylcobalamin 1000mcg/1mg
Folic Acid 400mcg
Contains NO added sugar, starch, wheat,
yeast, preservatives, artificial color or flavor.
Gluten Free.Not responsible for typographical errors. All errors subject to correction. These statements have not been evaluated by the FDA.
These products are not intended to diagnose, treat, cure or prevent any disease
$12.99 -
Sheer Adhesive Strips, Spots, 7/8, 100 / Box 017992808000
Sold By: Merit PharmaceuticalAdhesive Bandages
Fabric Adhesive Strips
Manufacturer # : 1307033 / 7615
Application : Adhesive Strip
Color : Tan
Dimensions : 7/8 SPOTS
Material : Plastic
Shape : Round
Sterility : SterileHighly absorbent non-stick pad to protect the wound
100% ultrasonic micro perforations to prevent skin maceration
Natural appearance
Transparent on the skin100 per box
$2.99 -
Sheer Adhesive Strips 1 X 3, 100 Box 017843200000
Sold By: Merit PharmaceuticalAdhesive Bandages
Fabric Adhesive Strips
Manufacturer # : 1290033
Application : Adhesive Strip
Color : Tan
Dimensions : 1 X 3 Inch
Material : Plastic
Shape : Rectangle
Sterility : SterileHighly absorbent non-stick pad to protect the wound
100% ultrasonic micro perforations to prevent skin maceration
Natural appearance
Transparent on the skin100 per box
$3.99 -
Fabric Adhesive Strips 1 X 3″, 100 Box 058459460000
Sold By: Merit PharmaceuticalFlexible Fabric Bandages
Fabric Adhesive Strips
Manufacturer # : 1595033
Application : Adhesive Strip
Color : Tan
Dimensions : 1 X 3 Inch
Material : Fabric
Shape : Rectangle
Sterility : SterileDesigned to provide comfortable protection
The soft weaved material bends easily and conforms to the body, staying on even when wet
The highly absorbent pad will not stick to the wound
Pre-folded wrapper tabs make them easy to open
Long lasting adhesive gently sticks to skin100 per box
$4.99 -
Transparent Film Dressing 2-3/8 X 2-3/4 Inch Frame Style MeriDerm 019713920000
Sold By: Merit PharmaceuticalBrand MeriDerm™
Manufacturer Merit Healthcare
Transparent Film Dressing
100/Box
Frame Style
Dimensions: 2-3/8 X 2-3/4 Inch
Octagon Shape
Sterile
Latex Free$43.99 -
Non-Sterile Dukal Cohesive Bandage 1″ Inch X 5 Yards Assorted Colors, 30/Box 088438030000
Sold By: Merit PharmaceuticalManufacturer # 8015AS
Manufacturer Dukal
Cohesive Bandage, Assorted Colors
Self-adherent Closure
1 Inch X 5 Yard
Non-Sterile
30/Box$28.99 -
Cohesive Bandage: Meritflex Latex Free, Assorted Colors (red, orange, teal, blue, brown) 2″ x 5 Yards 013843028000
Sold By: Merit PharmaceuticalMerit Healthcare # CB2540
Brand MeritFlex
Manufacturer Merit Healthcare
Application Cohesive Bandage
Closure Type Self-adherent Closure
Assorted Colors (brown, blue, red, orange, teal)
Dimensions 2 Inch X 5 Yard
Material Elastic with Cohesive
Sterility NonSterile
Latex Free40/Box
$36.00 -
Compressor Nebulizer – MeriMist 021128278250
Sold By: Merit PharmaceuticalIncluded In The Box:
- 1pc Medicine Cup
- 1pc Tubing 6.6ft Or 2m
- 1pc Adult Mask
- 1pc Pediatric Mask
- 1pc Mouthpiece
- 5pcs Filter
Easy To Use:
✓ RUNS QUIETLY
✓ SMALL, LIGHTWEIGHT (2.4 Lbs)
✓ COMPRESSOR FLOW: 8-10L
✓ RESERVOIR 8m$30.00 -
Covidien Uni-Patch Hot and Cold Reusable Gel Pack 013458615500
Sold By: Merit Pharmaceutical48 Packs to a Case
Durable construction for repeated use. Formulated with non-toxic and non-caustic ingredients.
Cold Therapy: Store in the freezer
Heat Therapy: CAREFULLY heat in hot water or microwave. Please follow the instructions.
Thick and flexible premium gel remains conformable at all temperatures.
Temperature Duration: 20 minutes.$36.99