- Medspa Essentials
- Aestheticians
- Apparel
- Clinic Furniture
- Devices
- Pharmaceutical & Medical Supplies
- Safety Supplies
- Training & Services
- Vendors
- Acara Partners
- Aesthetic Next
- Aesthetic Record
- American Med Spa Association
- Bonsai
- CEDR HR Solutions
- Colorescience
- CosmoGlo
- Derma Made
- Docovia
- Eden Skin And Body
- FACE Med Store
- KA Ring Designs
- LIFTIE Aspirator
- Massage Tools
- Merit Pharmaceutical
- National Medical Directors
- Post Love Skincare
- Rana Kennelley
- Source One Beauty
- SyringeRack
- The Aesthetic Immersion
- Velez by Vesna
- We Treat
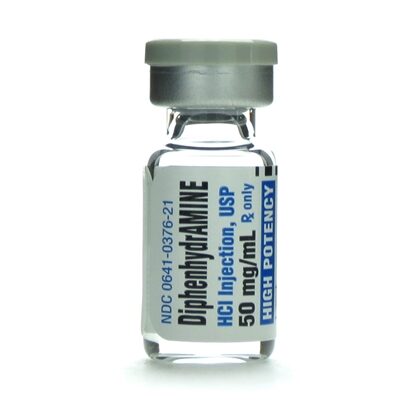
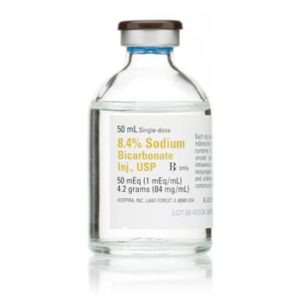
Diphenhydramine HCl 50 mg 029749515000
$44.75
Manufacturer: West-Ward Pharmaceuticals
Brand Generic Equivalent to Benadryl®
Application Antihistamine
Strength: 50mg/mL
Generic Equivalent to Diphenhydramine HCl
Vial
Generic Name
Storage Requirements USP Controlled Room Temperature
Type Intramuscular or Intravenous
Volume 1 mL
SKU
00641037625
Categories Pharmaceutical & Medical Supplies, Pharmacy, Prescription (Rx) Drugs & Supplies
Tags 50mg, Antihistamine, Benadryl, Diphenhydramine HCl, Intramuscular, Intravenous, Medical Supplies, Pharmacy, prescription, surgical supplies
Brand: West-Ward Pharmaceuticals
Diphenhydramine Hydrochloride Injection, USP 50 mg/mL is a sterile, nonpyrogenic solution intended for intramuscular or intravenous administration. Each 1 mL single-dose vial contains 50 mg of diphenhydramine HCl in water for injection, adjusted to a pH of 4.0–6.5 using sodium hydroxide or hydrochloric acid. This formulation is a generic equivalent to Benadryl®, offering high-potency antihistaminic relief in acute care settings.
Clinicians rely on diphenhydramine injection for rapid intervention in allergic reactions, anaphylaxis (as an adjunct to epinephrine), motion sickness, and mild cases of parkinsonism when oral therapy is impractical. Its anticholinergic and sedative properties make it a versatile agent in emergency and procedural environments.
This product is supplied in a box of 25 single-dose vials, making it ideal for hospitals, urgent care centers, medspas, and outpatient clinics. It is not intended for subcutaneous or intradermal use, and should never be used as a local anesthetic due to the risk of tissue necrosis.
Manufactured under strict quality standards, this injectable solution is latex-free, ensuring safety for patients with latex sensitivities. It should be stored at USP Controlled Room Temperature (20–25°C) and protected from light until use.
Diphenhydramine HCl injection is contraindicated in neonates, premature infants, and nursing mothers. Caution is advised in elderly patients and those with cardiovascular, respiratory, or neurological conditions. Adverse reactions may include drowsiness, dizziness, dry mouth, hypotension, and excitation in pediatric patients.
Shipping Countries: United States (US)
Ready to ship in 1-2 business days from United States (US)
Additional information
| Weight | N/A |
|---|---|
| Dimensions | N/A |
| options | Benadryl Each, Benadryl Box 25 |
-
$9.99 – $272.00Price range: $9.99 through $272.00Sold By: Merit Pharmaceutical
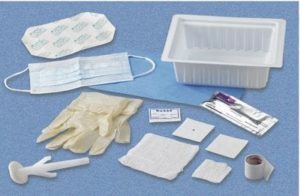
Central Line Tray With ChloraPrep One-Step Applicator 078121197500
$9.99 – $272.00Price range: $9.99 through $272.00 Select options -
$1.00 – $50.00Price range: $1.00 through $50.00Sold By: Merit Pharmaceutical
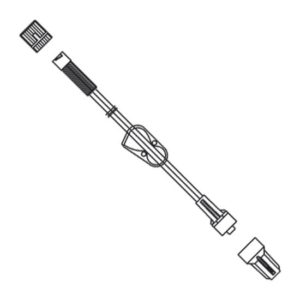
Extension Set, Minibore, 7”, Meritset 036001170000
$1.00 – $50.00Price range: $1.00 through $50.00 Select options -
$66.50
-
$1.99 – $62.50Price range: $1.99 through $62.50Sold By: Merit Pharmaceutical
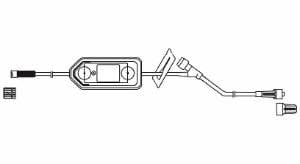
Extension Set, Regular Bore, 7”, Meriset 088713723000
$1.99 – $62.50Price range: $1.99 through $62.50 Select options
All pharmaceutical items are NONRETURNABLE.
Orders cancelled after shipment subject to a restocking fee.
Product Enquiry
Diphenhydramine Hydrochloride Injection, USP 50 mg/mL is a sterile, nonpyrogenic solution intended for intramuscular or intravenous administration. Each 1 mL single-dose vial contains 50 mg of diphenhydramine HCl in water for injection, adjusted to a pH of 4.0–6.5 using sodium hydroxide or hydrochloric acid. This formulation is a generic equivalent to Benadryl®, offering high-potency antihistaminic relief in acute care settings.
Clinicians rely on diphenhydramine injection for rapid intervention in allergic reactions, anaphylaxis (as an adjunct to epinephrine), motion sickness, and mild cases of parkinsonism when oral therapy is impractical. Its anticholinergic and sedative properties make it a versatile agent in emergency and procedural environments.
This product is supplied in a box of 25 single-dose vials, making it ideal for hospitals, urgent care centers, medspas, and outpatient clinics. It is not intended for subcutaneous or intradermal use, and should never be used as a local anesthetic due to the risk of tissue necrosis.
Manufactured under strict quality standards, this injectable solution is latex-free, ensuring safety for patients with latex sensitivities. It should be stored at USP Controlled Room Temperature (20–25°C) and protected from light until use.
Diphenhydramine HCl injection is contraindicated in neonates, premature infants, and nursing mothers. Caution is advised in elderly patients and those with cardiovascular, respiratory, or neurological conditions. Adverse reactions may include drowsiness, dizziness, dry mouth, hypotension, and excitation in pediatric patients.
Shipping Countries: United States (US)
Ready to ship in 1-2 business days from United States (US)
Additional information
| Weight | N/A |
|---|---|
| Dimensions | N/A |
| options | Benadryl Each, Benadryl Box 25 |
-
$9.99 – $272.00Price range: $9.99 through $272.00Sold By: Merit Pharmaceutical
Central Line Tray With ChloraPrep One-Step Applicator 078121197500
$9.99 – $272.00Price range: $9.99 through $272.00 Select options -
$1.00 – $50.00Price range: $1.00 through $50.00Sold By: Merit Pharmaceutical
Extension Set, Minibore, 7”, Meritset 036001170000
$1.00 – $50.00Price range: $1.00 through $50.00 Select options -
$66.50
-
$1.99 – $62.50Price range: $1.99 through $62.50Sold By: Merit Pharmaceutical
Extension Set, Regular Bore, 7”, Meriset 088713723000
$1.99 – $62.50Price range: $1.99 through $62.50 Select options
All pharmaceutical items are NONRETURNABLE.
Orders cancelled after shipment subject to a restocking fee.
Product Enquiry
Diphenhydramine Hydrochloride Injection, USP 50 mg/mL is a sterile, nonpyrogenic solution intended for intramuscular or intravenous administration. Each 1 mL single-dose vial contains 50 mg of diphenhydramine HCl in water for injection, adjusted to a pH of 4.0–6.5 using sodium hydroxide or hydrochloric acid. This formulation is a generic equivalent to Benadryl®, offering high-potency antihistaminic relief in acute care settings.
Clinicians rely on diphenhydramine injection for rapid intervention in allergic reactions, anaphylaxis (as an adjunct to epinephrine), motion sickness, and mild cases of parkinsonism when oral therapy is impractical. Its anticholinergic and sedative properties make it a versatile agent in emergency and procedural environments.
This product is supplied in a box of 25 single-dose vials, making it ideal for hospitals, urgent care centers, medspas, and outpatient clinics. It is not intended for subcutaneous or intradermal use, and should never be used as a local anesthetic due to the risk of tissue necrosis.
Manufactured under strict quality standards, this injectable solution is latex-free, ensuring safety for patients with latex sensitivities. It should be stored at USP Controlled Room Temperature (20–25°C) and protected from light until use.
Diphenhydramine HCl injection is contraindicated in neonates, premature infants, and nursing mothers. Caution is advised in elderly patients and those with cardiovascular, respiratory, or neurological conditions. Adverse reactions may include drowsiness, dizziness, dry mouth, hypotension, and excitation in pediatric patients.
Shipping Countries: United States (US)
Ready to ship in 1-2 business days from United States (US)
Additional information
| Weight | N/A |
|---|---|
| Dimensions | N/A |
| options | Benadryl Each, Benadryl Box 25 |
-
$9.99 – $272.00Price range: $9.99 through $272.00Sold By: Merit Pharmaceutical
Central Line Tray With ChloraPrep One-Step Applicator 078121197500
$9.99 – $272.00Price range: $9.99 through $272.00 Select options -
$1.00 – $50.00Price range: $1.00 through $50.00Sold By: Merit Pharmaceutical
Extension Set, Minibore, 7”, Meritset 036001170000
$1.00 – $50.00Price range: $1.00 through $50.00 Select options -
$66.50
-
$1.99 – $62.50Price range: $1.99 through $62.50Sold By: Merit Pharmaceutical
Extension Set, Regular Bore, 7”, Meriset 088713723000
$1.99 – $62.50Price range: $1.99 through $62.50 Select options
All pharmaceutical items are NONRETURNABLE.
Orders cancelled after shipment subject to a restocking fee.
Product Enquiry
Diphenhydramine Hydrochloride Injection, USP 50 mg/mL is a sterile, nonpyrogenic solution intended for intramuscular or intravenous administration. Each 1 mL single-dose vial contains 50 mg of diphenhydramine HCl in water for injection, adjusted to a pH of 4.0–6.5 using sodium hydroxide or hydrochloric acid. This formulation is a generic equivalent to Benadryl®, offering high-potency antihistaminic relief in acute care settings.
Clinicians rely on diphenhydramine injection for rapid intervention in allergic reactions, anaphylaxis (as an adjunct to epinephrine), motion sickness, and mild cases of parkinsonism when oral therapy is impractical. Its anticholinergic and sedative properties make it a versatile agent in emergency and procedural environments.
This product is supplied in a box of 25 single-dose vials, making it ideal for hospitals, urgent care centers, medspas, and outpatient clinics. It is not intended for subcutaneous or intradermal use, and should never be used as a local anesthetic due to the risk of tissue necrosis.
Manufactured under strict quality standards, this injectable solution is latex-free, ensuring safety for patients with latex sensitivities. It should be stored at USP Controlled Room Temperature (20–25°C) and protected from light until use.
Diphenhydramine HCl injection is contraindicated in neonates, premature infants, and nursing mothers. Caution is advised in elderly patients and those with cardiovascular, respiratory, or neurological conditions. Adverse reactions may include drowsiness, dizziness, dry mouth, hypotension, and excitation in pediatric patients.
Shipping Countries: United States (US)
Ready to ship in 1-2 business days from United States (US)
Additional information
| Weight | N/A |
|---|---|
| Dimensions | N/A |
| options | Benadryl Each, Benadryl Box 25 |
-
$9.99 – $272.00Price range: $9.99 through $272.00Sold By: Merit Pharmaceutical
Central Line Tray With ChloraPrep One-Step Applicator 078121197500
$9.99 – $272.00Price range: $9.99 through $272.00 Select options -
$1.00 – $50.00Price range: $1.00 through $50.00Sold By: Merit Pharmaceutical
Extension Set, Minibore, 7”, Meritset 036001170000
$1.00 – $50.00Price range: $1.00 through $50.00 Select options -
$66.50
-
$1.99 – $62.50Price range: $1.99 through $62.50Sold By: Merit Pharmaceutical
Extension Set, Regular Bore, 7”, Meriset 088713723000
$1.99 – $62.50Price range: $1.99 through $62.50 Select options
All pharmaceutical items are NONRETURNABLE.
Orders cancelled after shipment subject to a restocking fee.
Product Enquiry
Diphenhydramine Hydrochloride Injection, USP 50 mg/mL is a sterile, nonpyrogenic solution intended for intramuscular or intravenous administration. Each 1 mL single-dose vial contains 50 mg of diphenhydramine HCl in water for injection, adjusted to a pH of 4.0–6.5 using sodium hydroxide or hydrochloric acid. This formulation is a generic equivalent to Benadryl®, offering high-potency antihistaminic relief in acute care settings.
Clinicians rely on diphenhydramine injection for rapid intervention in allergic reactions, anaphylaxis (as an adjunct to epinephrine), motion sickness, and mild cases of parkinsonism when oral therapy is impractical. Its anticholinergic and sedative properties make it a versatile agent in emergency and procedural environments.
This product is supplied in a box of 25 single-dose vials, making it ideal for hospitals, urgent care centers, medspas, and outpatient clinics. It is not intended for subcutaneous or intradermal use, and should never be used as a local anesthetic due to the risk of tissue necrosis.
Manufactured under strict quality standards, this injectable solution is latex-free, ensuring safety for patients with latex sensitivities. It should be stored at USP Controlled Room Temperature (20–25°C) and protected from light until use.
Diphenhydramine HCl injection is contraindicated in neonates, premature infants, and nursing mothers. Caution is advised in elderly patients and those with cardiovascular, respiratory, or neurological conditions. Adverse reactions may include drowsiness, dizziness, dry mouth, hypotension, and excitation in pediatric patients.
Shipping Countries: United States (US)
Ready to ship in 1-2 business days from United States (US)
Additional information
| Weight | N/A |
|---|---|
| Dimensions | N/A |
| options | Benadryl Each, Benadryl Box 25 |
-
$9.99 – $272.00Price range: $9.99 through $272.00Sold By: Merit Pharmaceutical
Central Line Tray With ChloraPrep One-Step Applicator 078121197500
$9.99 – $272.00Price range: $9.99 through $272.00 Select options -
$1.00 – $50.00Price range: $1.00 through $50.00Sold By: Merit Pharmaceutical
Extension Set, Minibore, 7”, Meritset 036001170000
$1.00 – $50.00Price range: $1.00 through $50.00 Select options -
$66.50
-
$1.99 – $62.50Price range: $1.99 through $62.50Sold By: Merit Pharmaceutical
Extension Set, Regular Bore, 7”, Meriset 088713723000
$1.99 – $62.50Price range: $1.99 through $62.50 Select options
All pharmaceutical items are NONRETURNABLE.
Orders cancelled after shipment subject to a restocking fee.
Product Enquiry
Diphenhydramine Hydrochloride Injection, USP 50 mg/mL is a sterile, nonpyrogenic solution intended for intramuscular or intravenous administration. Each 1 mL single-dose vial contains 50 mg of diphenhydramine HCl in water for injection, adjusted to a pH of 4.0–6.5 using sodium hydroxide or hydrochloric acid. This formulation is a generic equivalent to Benadryl®, offering high-potency antihistaminic relief in acute care settings.
Clinicians rely on diphenhydramine injection for rapid intervention in allergic reactions, anaphylaxis (as an adjunct to epinephrine), motion sickness, and mild cases of parkinsonism when oral therapy is impractical. Its anticholinergic and sedative properties make it a versatile agent in emergency and procedural environments.
This product is supplied in a box of 25 single-dose vials, making it ideal for hospitals, urgent care centers, medspas, and outpatient clinics. It is not intended for subcutaneous or intradermal use, and should never be used as a local anesthetic due to the risk of tissue necrosis.
Manufactured under strict quality standards, this injectable solution is latex-free, ensuring safety for patients with latex sensitivities. It should be stored at USP Controlled Room Temperature (20–25°C) and protected from light until use.
Diphenhydramine HCl injection is contraindicated in neonates, premature infants, and nursing mothers. Caution is advised in elderly patients and those with cardiovascular, respiratory, or neurological conditions. Adverse reactions may include drowsiness, dizziness, dry mouth, hypotension, and excitation in pediatric patients.
Shipping Countries: United States (US)
Ready to ship in 1-2 business days from United States (US)
Additional information
| Weight | N/A |
|---|---|
| Dimensions | N/A |
| options | Benadryl Each, Benadryl Box 25 |
-
$9.99 – $272.00Price range: $9.99 through $272.00Sold By: Merit Pharmaceutical
Central Line Tray With ChloraPrep One-Step Applicator 078121197500
$9.99 – $272.00Price range: $9.99 through $272.00 Select options -
$1.00 – $50.00Price range: $1.00 through $50.00Sold By: Merit Pharmaceutical
Extension Set, Minibore, 7”, Meritset 036001170000
$1.00 – $50.00Price range: $1.00 through $50.00 Select options -
$66.50
-
$1.99 – $62.50Price range: $1.99 through $62.50Sold By: Merit Pharmaceutical
Extension Set, Regular Bore, 7”, Meriset 088713723000
$1.99 – $62.50Price range: $1.99 through $62.50 Select options
All pharmaceutical items are NONRETURNABLE.
Orders cancelled after shipment subject to a restocking fee.
Product Enquiry
Diphenhydramine Hydrochloride Injection, USP 50 mg/mL is a sterile, nonpyrogenic solution intended for intramuscular or intravenous administration. Each 1 mL single-dose vial contains 50 mg of diphenhydramine HCl in water for injection, adjusted to a pH of 4.0–6.5 using sodium hydroxide or hydrochloric acid. This formulation is a generic equivalent to Benadryl®, offering high-potency antihistaminic relief in acute care settings.
Clinicians rely on diphenhydramine injection for rapid intervention in allergic reactions, anaphylaxis (as an adjunct to epinephrine), motion sickness, and mild cases of parkinsonism when oral therapy is impractical. Its anticholinergic and sedative properties make it a versatile agent in emergency and procedural environments.
This product is supplied in a box of 25 single-dose vials, making it ideal for hospitals, urgent care centers, medspas, and outpatient clinics. It is not intended for subcutaneous or intradermal use, and should never be used as a local anesthetic due to the risk of tissue necrosis.
Manufactured under strict quality standards, this injectable solution is latex-free, ensuring safety for patients with latex sensitivities. It should be stored at USP Controlled Room Temperature (20–25°C) and protected from light until use.
Diphenhydramine HCl injection is contraindicated in neonates, premature infants, and nursing mothers. Caution is advised in elderly patients and those with cardiovascular, respiratory, or neurological conditions. Adverse reactions may include drowsiness, dizziness, dry mouth, hypotension, and excitation in pediatric patients.
Shipping Countries: United States (US)
Ready to ship in 1-2 business days from United States (US)
Additional information
| Weight | N/A |
|---|---|
| Dimensions | N/A |
| options | Benadryl Each, Benadryl Box 25 |
-
$9.99 – $272.00Price range: $9.99 through $272.00Sold By: Merit Pharmaceutical
Central Line Tray With ChloraPrep One-Step Applicator 078121197500
$9.99 – $272.00Price range: $9.99 through $272.00 Select options -
$1.00 – $50.00Price range: $1.00 through $50.00Sold By: Merit Pharmaceutical
Extension Set, Minibore, 7”, Meritset 036001170000
$1.00 – $50.00Price range: $1.00 through $50.00 Select options -
$66.50
-
$1.99 – $62.50Price range: $1.99 through $62.50Sold By: Merit Pharmaceutical
Extension Set, Regular Bore, 7”, Meriset 088713723000
$1.99 – $62.50Price range: $1.99 through $62.50 Select options
All pharmaceutical items are NONRETURNABLE.
Orders cancelled after shipment subject to a restocking fee.
Product Enquiry
Best selling
-
Easy Touch Insulin
$15.99 -
Non-Woven Sponges
$3.50 -
IV Start Kit Latex Free
$77.00
-
-
Sharps Container
$5.25
-
Related Products
Related Products
-
$10.00 – $100.00Price range: $10.00 through $100.00Sold By: Merit Pharmaceutical
Isolation Gown, Yellow, Polyethylene, 10 per pack 013026040843
$10.00 – $100.00Price range: $10.00 through $100.00 Select options
-
$10.00 – $100.00Price range: $10.00 through $100.00Sold By: Merit Pharmaceutical
Isolation Gown, Yellow, Polyethylene, 10 per pack 013026040843
$10.00 – $100.00Price range: $10.00 through $100.00 Select options
-
$650.00
$850.00Sold By: Rana KennelleyTwo Accredited Neuromodulator On-Line Training Courses
$650.00$850.00Add to cart -
$790.00
$990.00Sold By: Rana KennelleyTwo Accredited Facial Filler On-Line Training Courses
$790.00$990.00Add to cart -
$91.00
$113.99 -
Manual Bundle
Sold By: Acara Partners$1,250.00$1,750.00 -
Collins Cameo Reception Desk with 10 Color Options ($1 Shipping) Made in USA
Sold By: Source One Beauty$1,009.00$1,062.00Sold By: Source One BeautyCollins Cameo Reception Desk with 10 Color Options ($1 Shipping) Made in USA
$1,009.00$1,062.00Select options -
Collins Reve Concierge Desk with 10 Color Options ($1 Shipping) Made in USA
Sold By: Source One Beauty$1,031.50$1,086.00Sold By: Source One BeautyCollins Reve Concierge Desk with 10 Color Options ($1 Shipping) Made in USA
$1,031.50$1,086.00Select options -
Collins Reve Retail Display with 10 Color Options ($1 Shipping) Made in USA
Sold By: Source One Beauty$838.00$882.00 -
Collins Amati Retail Display with 10 Color Options ($1 Shipping) Made in USA
Sold By: Source One Beauty$792.50$834.00 -
$2,462.50
$2,592.00
-
$650.00
$850.00Sold By: Rana KennelleyTwo Accredited Neuromodulator On-Line Training Courses
$650.00$850.00Add to cart -
$790.00
$990.00Sold By: Rana KennelleyTwo Accredited Facial Filler On-Line Training Courses
$790.00$990.00Add to cart -
$91.00
$113.99 -
Manual Bundle
Sold By: Acara Partners$1,250.00$1,750.00 -
Collins Cameo Reception Desk with 10 Color Options ($1 Shipping) Made in USA
Sold By: Source One Beauty$1,009.00$1,062.00Sold By: Source One BeautyCollins Cameo Reception Desk with 10 Color Options ($1 Shipping) Made in USA
$1,009.00$1,062.00Select options -
Collins Reve Concierge Desk with 10 Color Options ($1 Shipping) Made in USA
Sold By: Source One Beauty$1,031.50$1,086.00Sold By: Source One BeautyCollins Reve Concierge Desk with 10 Color Options ($1 Shipping) Made in USA
$1,031.50$1,086.00Select options -
Collins Reve Retail Display with 10 Color Options ($1 Shipping) Made in USA
Sold By: Source One Beauty$838.00$882.00 -
Collins Amati Retail Display with 10 Color Options ($1 Shipping) Made in USA
Sold By: Source One Beauty$792.50$834.00 -
$2,462.50
$2,592.00