Preservative Free
-
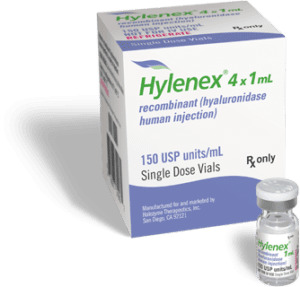
Hylenex Package: Hylenex Hyaluronidase, Human Recombinant, Preservative Free 150 Unit / mL Injection Single Dose Vial Box of 4 x 1 mL
Hylenex® recombinant human hyaluronidase enhances the absorption and dispersion of injected drugs and fluids, supporting efficient, predictable results in medical and aesthetic procedures.
$379.99 -
Ropivacaine HCl, Preservative Free 5mg/ml 150mg/30ml 1 x 10 NDC 032221791375
Ropivacaine HCl, Preservative Free5mg/ml 150mg/30ml 1 x 10 (Box of 10 Vials)
Infiltration, Nerve Block and Epidural Injection Single Dose Vial 10 mL
NDC# 0409-9301-30 / 00409930130 / 0409930130Non-Returnable
$104.99 -
Sterile Water for Injection
Sterile Water for Injection, Preservative Free USP
Application: Diluent
Single Dose Fliptop Vial
Non-DEHP
Preservative Free
Latex Free: Latex Information: Natural rubber latex has not been used in the manufacture of this device or drug container closure system
Manufacturer: Hospira$103.99 -
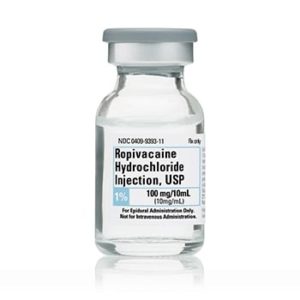
Ropivacaine HCl, Preservative Free 10mg/ml 100mg/10mL 1 x 10 NDC
Ropivacaine HCl, Preservative Free 7.5mg/ml 150mg/20mL Box of 1 x 10 (10 vials)
Infiltration, Nerve Block and Epidural Injection Single Dose Vial 10 mL
NDC# 0409-9303-10 / 0409930310 / 00409-9303-10Non-Returnable
$54.99 -
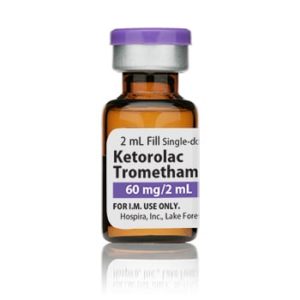
Ketorolac Tromethamine Injection
Manufacturer: Hospira
Application Nonsteroidal Anti-inflammatory Agent
Strength: 30mg/mL
Generic Ketorolac Tromethamine, Preservative Free
Single Dose Vial
Storage Requirements USP Controlled Room Temperature
Latex Information indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure systemPrice range: $49.99 through $54.99 -
BD PosiFlush Heparin Sodium 030565600500
NDC Number 08290-3064-24 / 08290306424 / 306424
Manufacturer: BD – Becton DickinsonReorder # 306424
Application Heparin Lock Flush
Strength: 05%, 5mg/mL
Generic Heparin Sodium, Porcine, Preservative Free
Prefilled Syringe
Storage Requirements USP Controlled Room Temperature
Type Intravenous
Volume 5 mL$31.99 -
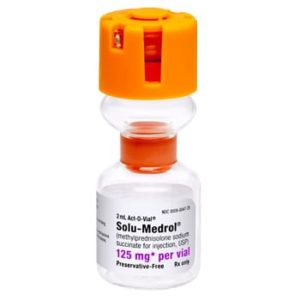
Solu-Medrol Methylprednisolone Sodium Succinate 032336805000
NDC Number 00009-0047-22 / 0009004722
Manufacturer Pfizer
Brand Solu-Medrol®
Application Corticosteroid
Strength: 125mg/2mL
Generic Equivalent to Marcaine®, Sensorcaine®
Single Dose Vial – Act-O-Vial
Storage Requirements USP Controlled Room Temperature
Volume 2 mL$14.99 -
Solu-Medrol Methylprednisolone Sodium Succinate 40MG 032365935000
NDC Number 00009-00039-28 / 00009003928
Brand Solu-Medrol®
Manufacturer: Pfizer
Application Corticosteroid
Strength: 40mg/mL
Generic Equivalent to Methylprednisolone Sodium Succinate, Preservative Free
Single Dose Vial
Storage Requirements USP Controlled Room Temperature
Type Intramuscular, Intravenous, or Subcutaneous
Volume 1 mL$9.99 -
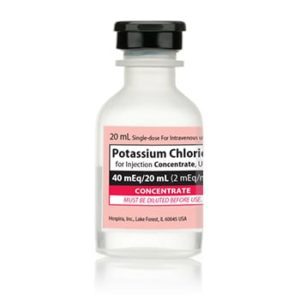
Potassium Chloride Concentrate 030827238000
NDC Number 00409-6653-05 / 00409665305
Manufacturer: Hospira
Application Replacement Preparation
Strength: 2 mEq/mL
Generic Equivalent to Marcaine®, Sensorcaine®
Single Dose Vial
Storage Requirements USP Controlled Room Temperature
Type Intravenous
Volume 20 mL
Latex Information indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system$9.50