antibodies
-
Mononucleosis Test 20 Per Box
PROA-P080016
Rapid chromatographic immunoassay technology identifies heterophile antibodies in whole blood, serum or plasma to aid in the diagnosis of Infectious Mononucleosis.
• Qualitative membrane strip based immunoassay for detection
of IM heterophile antibodies
• All inclusive 20 test kit
• Simple to perform using whole blood (from venipucture or
fingerstick), serum or plasma
• Reliable results in 5 minutes
• CLIA waived for whole blood and FDA 510(k) cleared to marketIncludes 20 Individually Packaged Mono Test Devices, 20 Disposable Sample Droppers, 20 Disposable Heparinized Capillary Tubes, 1 Dispensing Bulb, 1 Sample Buffer, 1 Procedure Card, 1 Package Insert, 20 tst/bx. CLIA Waived.
$55.00 -
H. Pylori Test Device 20/Bx 012898152462
H. Pylori Test Device Kit, 20 Tubes
One-step, qualitative membrane strip-based immunoassay designed for detection of H. pylori IgG antibodies in whole blood, serum or plasma
Test results in 10 minutes helps assist physicians in making treatment decisions during office visit
88% sensitivity with 89% specificity
CLIA waived for whole bloodIncludes:
- 20 Individually Packaged H.pylori Test Devices
- Disposable Sample Droppers
- 20 Disposable Heparinized Capillary Tubes & Dispensing Bulk
- 1 Positive Control
- 1 Negative Control
- 1 Sample Buffer
- 20 test/kit
$105.00 -
Orasure Quickflu Rapid Flu A & B Test, 22 Tests Box 013602705375
Orasure Quickflu Rapid Flu A & B Test, 22 Tests/Bx
# 1001-0320
Non-ReturnableThe OraQuick® HCV test is FDA approved for detecting HCV antibodies in fingerstick and venipuncture whole blood. Our simple platform enables healthcare providers to deliver an accurate diagnosis in 20 minutes.
SENSITIVITY TO MEET YOUR NEEDS
OraSure QuickFlu® is an FDA cleared, CLIA-waived in vitro rapid qualitative test for the detection and differentiation of influenza type A and type B. An easy nasal swab procedure provides accurate results in as little as 10 minutes.
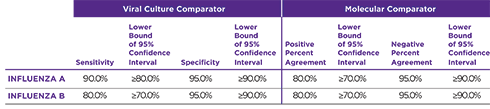
Orasure QuickFlu® delivers highly accurate results for flu types A and B and meets FDA’s new standards of performance.
- Proven analytical sensitivity across 51 different flu strains including CDC’s 2019-2020 flu strain panels
- Exceptional correlation to culture/PCR
PROVEN PERFORMANCE THAT MEETS FDA’S RECLASSIFICATION RULES
OraSure QuickFlu® exceeds the FDA’s reclassification requirement minimums for RIDTs.
- As of February 13, 2017, all RIDTs must meet the minimum acceptance performance criteria and comparator method for antigen based RIDTs. Tests that do not meet the new requirements cannot be used.
- Analytical reactivity testing must be performed on contemporary influenza strains and newly emerging strains in the event of an emergency.
ENSURES RESPONSIVE STANDARD OF CARE
-
- Simple, easy-to-use procedure with built-in control line
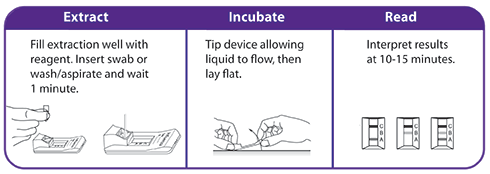
- Includes a flocked swab that optimizes sample collection and release resulting in superior performance
- American Society of Microbiology says, “NP flocked swabs collect better samples than routine NP swabs”
- Nasal swab collection – comfortable for the patient and simple for the HCP with no ancillary supplies required
- Ready-to-use reagents with room temperature storage
- Rapid testing that maximizes results
TEST AT THE POINT OF CARE
OraSure QuickFlu® is ideal for testing at the point of care in…
- Laboratories
- Emergency Rooms
- Physician Offices
- Public Health Facilities and Clinics
REIMBURSEMENT INFORMATION
CPT Codes
87804 QW- Influenza A Detection
87804-59- Influenza B Detection
$318.99