Intravenous
-
B Braun IV Solutions Sterile Water Injection USP B Braun IV Solutions
Generic Drug Name Sterile Water for Injection, Preservative Free
Manufacturer # L8501-01
Manufacturer B. Braun
Application Diluent
Container Type Flexible Bag
Dosage Form IV Solution
NDC Number 00264-7850-10
Type Intravenous
Size Volume 500 mLNot manufactured with latex, PVC or DEHP.
Price range: $7.00 through $127.00
B Braun IV Solutions Sterile Water Injection USP B Braun IV Solutions
Price range: $7.00 through $127.00 Select options -
Potassium Chloride Concentrate 030827238000
NDC Number 00409-6653-05 / 00409665305
Manufacturer: Hospira
Application Replacement Preparation
Strength: 2 mEq/mL
Generic Equivalent to Marcaine®, Sensorcaine®
Single Dose Vial
Storage Requirements USP Controlled Room Temperature
Type Intravenous
Volume 20 mL
Latex Information indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system$9.50 -
Ondansetron HCL, MDV 2mg/mL 20mL 032158586812
NDC Number 16729-0298-05 / 16729029805
Manufacturer: Accord Healthcare
Application Prevention of Nausea and Vomiting
Strength: 2mg/mL
Multiple Dose Vial
Storage Requirements USP Controlled Room Temperature
Type Intramuscular, Intravenous, or Subcutaneous
Volume 20 mL
Latex Information indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system$9.99 -
Sodium Bicarbonate 024916906832
Sterile sodium bicarbonate 8.4% injection USP for clinical use. Preservative-free solution for metabolic acidosis correction and pH adjustment in medical settings.
Price range: $18.99 through $425.00 -
Dexamethasone Sodium Phosphate 029643576000
Dexamethasone Sodium Phosphate 4 mg / mL Intramuscular or Intravenous Injection Multiple Dose Vial 30 mL
NDC Number 6745742130 / 67457-421-30
Manufacturer: Mylan Institutional
Application Corticosteroid
Strength: 4mg/mL
Multiple Dose Vial
Generic Name Dexamethasone Sodium Phosphate
Storage Requirements USP Controlled Room Temperature
Type Intramuscular or Intravenous / Injection
Volume 30 mL
Latex Information indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system$23.70 -
Chromium (Chromic Chloride Injection, USP) 028745525000
NDC Number 0040909301
Manufacturer: Hospira
Strength: 4mcg/mL
Generic Equivalent to Marcaine®, Sensorcaine®
Single Dose Vial
Generic Name Chromic Chloride
Storage Requirements USP Controlled Room Temperature
Type Intravenous
Volume 10 mL
Latex Information indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system.$30.00 -
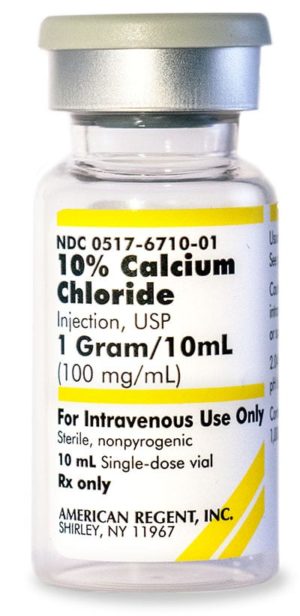
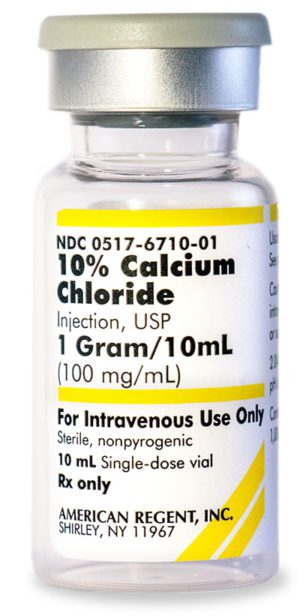
10% Calcium Chloride Injection, USP 028867198000
NDC Number 0517-6710-10
NDC Number 00517671010
Manufacturer: American Regent
Application Replacement Preparation
Strength: 100mg/mL
Single Dose Vial
Storage Requirements USP Controlled Room Temperature
Type Intravenous
Volume 10 mLPrice range: $33.99 through $339.90 -
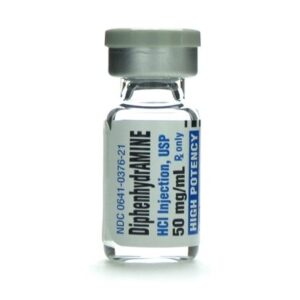
Diphenhydramine HCl 50 mg 029749515000
Manufacturer: West-Ward Pharmaceuticals
Brand Generic Equivalent to Benadryl®
Application Antihistamine
Strength: 50mg/mL
Generic Equivalent to Diphenhydramine HCl
Vial
Generic Name
Storage Requirements USP Controlled Room Temperature
Type Intramuscular or Intravenous
Volume 1 mL$44.75 -
Plenamine 15% Amino Acids Injection 1000 mL 026346274750
Latex Free, DEHP Free BAG
Per the manufacturer: Acceptable Dating: >= 90 days
Pharmacy Bulk Package is a sterile, clear, nonpyrogenic solution of essential and nonessential amino acids for intravenous infusion in parenteral nutrition following appropriate dilution.
Plenamine™ 15% in a Pharmacy Bulk Package is not for direct infusion. It is a sterile dosage form which contains several single doses for use in a pharmacy admixture program in the preparation of intravenous parenteral fluids.
Non-Returnable.
$51.99 -
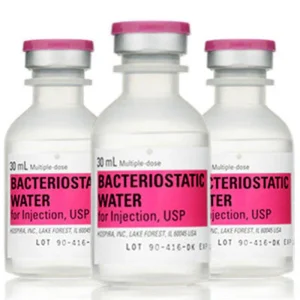
Bacteriostatic Water for Injection Intramuscular 029450054500 | 30mL Vials, 25-Pack
NDC Number 00409-3977-03
NDC 00409397703
Manufacturer: Hospira
Application Diluent
Container Type Multiple Dose Vial
Generic Drug Name Bacteriostatic Water for Injection
Storage Requirements USP Controlled Room Temperature
Type Intramuscular, Intravenous, or Subcutaneous$289.99