USP
-
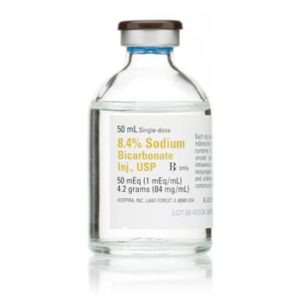
Sodium Bicarbonate 024916906832
Sterile sodium bicarbonate 8.4% injection USP for clinical use. Preservative-free solution for metabolic acidosis correction and pH adjustment in medical settings.
Price range: $18.99 through $425.00 -
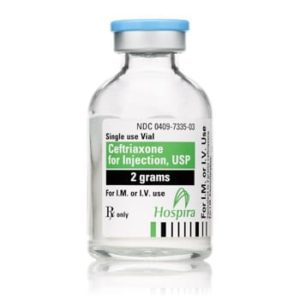
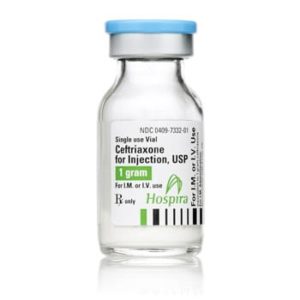
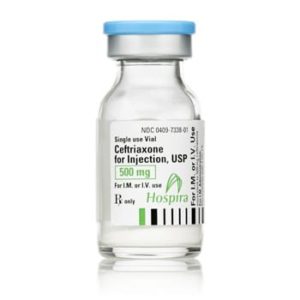
Ceftriaxone for Injection
Ceftriaxone for Injection, USP
Brand Name Equivalent: Rocephin®
Manufacturer Hospira/Pfizer
15mL Single Dose Vial“Latex Information” indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system.
“Preservative Information” indicates that this product does not contain ingredients identified as a preservative, as defined by the USP.
Price range: $21.00 through $53.00 -
Chromium (Chromic Chloride Injection, USP) 028745525000
NDC Number 0040909301
Manufacturer: Hospira
Strength: 4mcg/mL
Generic Equivalent to Marcaine®, Sensorcaine®
Single Dose Vial
Generic Name Chromic Chloride
Storage Requirements USP Controlled Room Temperature
Type Intravenous
Volume 10 mL
Latex Information indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system.$30.00 -
Lidocaine 2%, 20mg/mL 50 mL 031386446000
Manufacturer: Hospira
Brand Generic Equivalent to Xylocaine®
Application Local Anesthetic
Strength: 2%, 20mg/mL
Generic Lidocaine HCl
Multiple Dose Vial
Storage Requirements USP Controlled Room Temperature
Type Injection, Inflitration and Nerve Block
Volume 50 mL
Latex Informationindicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system$6.99