Merit Pharmaceutical
- 2611 N. San Fernando Road, Los Angeles, California, United States (US)
Please call Merit Customer Support at 800.69.MERIT. A live person will answer the phone and help you Monday – Friday 7am – 5:00pm PST. Or reach us by e-mail: solutions@meritpharm.com.
solutions@meritpharm.com-
Cephalexin 500mg Bottle 100 019551319125
Cephalexin
Common brand names: KeflexThis medication is used to treat a wide variety of bacterial infections.
Brand Names: Keflex · Daxbia · Zartan · Biocef · Panixine DisperDose and more
Drug Class: Cephalosporin Antibiotics – 1st Generation
Manufacturer Varies
Non-Returnable
#019551319125
$14.35 -
Amoxicillin 500 mg Capsule Bottle 100 024137751000
Amoxicillin may be used to treat a wide variety of bacterial infections.
Manufacturer Varies
Non-Returnable
$16.99 -
Extension Set, Minibore, 7”, Meritset 036001170000
Manufacturer # EX001
Manufacturer Merit Healthcare
Needle Free Extension Set, Minibore
Regular Bore Tubing OD approximately 4.0ml
Connection Type : 2 Rotating Luer Lock Connector and Cap. Male/Female Luer Slide Clamp and Cap
Flow Control Type Clamp
Tubing Length 7 Inch Tubing (approximately).
Capacity Approx. 0.7mL
Tubing Type: DEHP-Free/LATEX FREE/Pyrogen Free$1.00 – $50.00 -
Handheld Aneroid Sphygmomanometer
Manufacturer American Diagnostic Corp
Blue Color, Nylon Cuff
Latex Free$18.99 – $31.99 -
Lidocaine/Prilocaine (Generic EMLA) 2.5%, 30GM NDC # 00168-0357-30 027877917515
Lidocaine / Prilocaine Topical (Generic EMLA)
Lidocaine 2.5% and Prilocaine 2.5% Cream, 30 g
RX onlyNON-RETURNABLE
#027857236031
$30.00 -
Sundry Jars, Unlabeled, Size: 7″ x 4 1/4″ 6 Jars Per Case 026868540972
Provides exam room storage for products such as bandages, gauze, cotton, applicators, and tongue depressors
Flint glass
Not autoclavable
Stainless steel lidSold Case of 6
$45.99 -
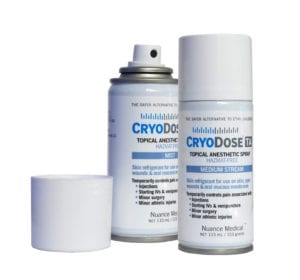
Cryodose TA Topical Anesthetic Spray 010073655312
CryoDose TA: Now you (and patients) can benefit from a non-toxic topical anesthetic spray that is a clinical and therapeutic equivalent to hazardous ethyl chloride – for use on skin, minor open wounds & intact oral mucous membranes.
CryoDose TA temporarily controls pain associated with injections, starting IVs, venipuncture, minor surgical procedures, and minor athletic injuries.
So many reasons to convert to CryoDose TA skin refrigerant:
Safer alternative to ethyl chloride
FDA clearance as “substantially equivalent” to ethyl chloride
Dosage, use, effect, duration are identical
More uses: FDA indications includes minor open woundsHAZMAT-free
Non-flammable
No exceptional inhalation risks
Available in Mist Spray or Medium Stream
100% satisfaction guarantee$49.99 -
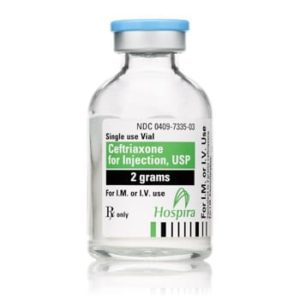
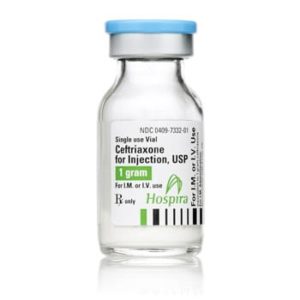
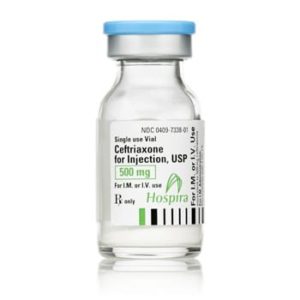
Ceftriaxone for Injection
Ceftriaxone for Injection, USP
Brand Name Equivalent: Rocephin®
Manufacturer Hospira/Pfizer
15mL Single Dose Vial“Latex Information” indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system.
“Preservative Information” indicates that this product does not contain ingredients identified as a preservative, as defined by the USP.
$21.00 – $53.00 -
Insulin Syringe with Needle 1/3cc 29G x 1/2 / 0.3 mL (1/3 cc) 29 Gauge 1/2 Inch 042892157000
Manufacturer # 26018
Manufacturer / Brand Exel International
1/3cc 29G x 5/19
Non-Safety
Attached Needle
100 Per Box, 5 Packs of 10
Case = 5 x 100
Latex Free$15.99 -
Methylcobalamin 1000mcg / 1mg Cherry Flavor, 60 Sublingual Lozenges 006313560000
Each 1 Lozenge:
Methylcobalamin 1000mcg/1mg
Folic Acid 400mcg
Contains NO added sugar, starch, wheat,
yeast, preservatives, artificial color or flavor.
Gluten Free.Not responsible for typographical errors. All errors subject to correction. These statements have not been evaluated by the FDA.
These products are not intended to diagnose, treat, cure or prevent any disease
$12.99 -
KN95 Disposable Face Mask, White 25 Per Box 081576852500
KN95 Mask, Facial Mask with earloop.
25 Per Box
White. One size fits most.
Manufacturer: Medigrative
Latex Free
Material: Spunbond Polypropylene
Color: White
Used For: Healthcare / Dental / Laboratory / Homecare / Industrial Safety / Foodservice$37.50 -
3mL 20G x 1″, 3cc Syringe, Luer Lock 043193323000
Manufacturer Number: 26108
Manufacturer: Exel International
Volume: 3mL
Gauge: 20 Gauge Needle
Needle Length: 1”
Graduations: 0.5mL Increments
Application: Syringe with Hypodermic Needle
Needle Material Stainless Steel
Needle Point Style Regular Bevel
Needle Style Detachable Needle
Sterile: Yes
Type: Non-Safety
Connection Type: Standard Luer-Lock Tip
Packaging: Blister Pack
Cap: No Cap
Syringe Material: Polypropylene
Tip Type: Luer Lock Tip
Latex Free
Packaged: 100 Per Box, 10 Boxes Per Case$18.99