Room Temperature
-
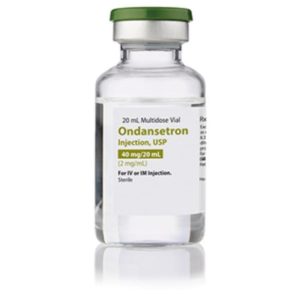
Ondansetron HCL, MDV 2mg/mL 20mL
NDC Number 16729-0298-05 / 16729029805
Manufacturer: Accord Healthcare
Application Prevention of Nausea and Vomiting
Strength: 2mg/mL
Multiple Dose Vial
Storage Requirements USP Controlled Room Temperature
Type Intramuscular, Intravenous, or Subcutaneous
Volume 20 mL
Latex Information indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system$9.99 -
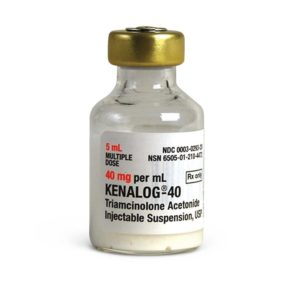
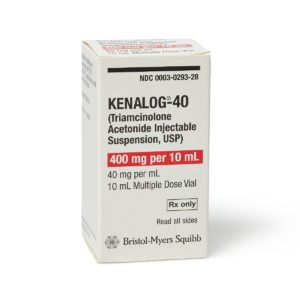
Kenalog
Manufacturer: Bristol-Myers Squibb
Brand Kenalog®-40
Application Corticosteroid
Generic Equivalent to Marcaine®, Sensorcaine®
Multiple Dose Vial
Storage Requirements USP Controlled Room Temperature
Type Injectable / Injection / Intramuscular or Intra-Asticular$20.99 – $101.99 -
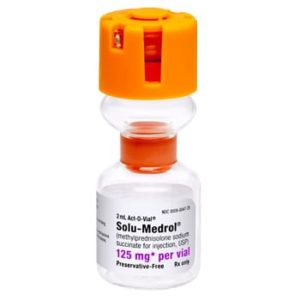
Solu-Medrol Methylprednisolone Sodium Succinate
NDC Number 00009-0047-22 / 0009004722
Manufacturer Pfizer
Brand Solu-Medrol®
Application Corticosteroid
Strength: 125mg/2mL
Generic Equivalent to Marcaine®, Sensorcaine®
Single Dose Vial – Act-O-Vial
Storage Requirements USP Controlled Room Temperature
Volume 2 mL$14.99 -
Sodium Chloride Flush Syringes 120/CASE
Application Flush Syringe
Strength: 0.9%
Single Use Syringe
Storage Requirements USP Controlled Room Temperature
Volume 10 mL$89.99 -
Hibiclens 16 oz
Brand Hibiclens®
Manufacturer Molnlycke
4% Strength
Active Ingredients CHG (Chlorhexidine Gluconate)
Surgical Scrub
16 oz Bottle
Storage Requirements USP Controlled Room Temperature$14.99 -
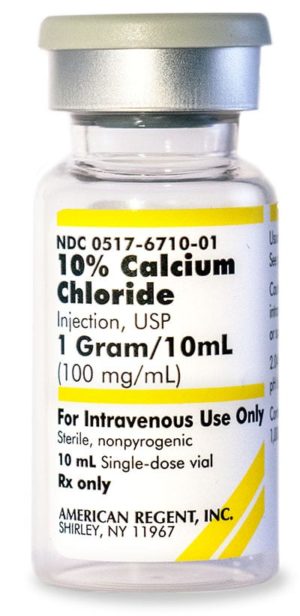
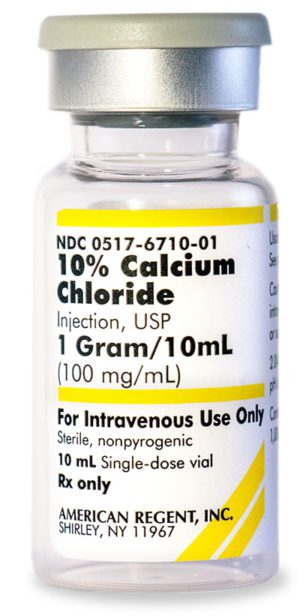
10% Calcium Chloride Injection, USP
NDC Number 0517-6710-10
NDC Number 00517671010
Manufacturer: American Regent
Application Replacement Preparation
Strength: 100mg/mL
Single Dose Vial
Storage Requirements USP Controlled Room Temperature
Type Intravenous
Volume 10 mL$33.99 – $339.90 -
Dexamethasone Sodium Phosphate
Dexamethasone Sodium Phosphate 4 mg / mL Intramuscular or Intravenous Injection Multiple Dose Vial 30 mL
NDC Number 6745742130 / 67457-421-30
Manufacturer: Mylan Institutional
Application Corticosteroid
Strength: 4mg/mL
Multiple Dose Vial
Generic Name Dexamethasone Sodium Phosphate
Storage Requirements USP Controlled Room Temperature
Type Intramuscular or Intravenous / Injection
Volume 30 mL
Latex Information indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system$23.70 -
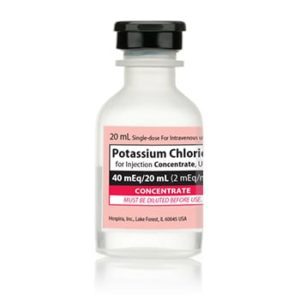
Potassium Chloride Concentrate
NDC Number 00409-6653-05 / 00409665305
Manufacturer: Hospira
Application Replacement Preparation
Strength: 2 mEq/mL
Generic Equivalent to Marcaine®, Sensorcaine®
Single Dose Vial
Storage Requirements USP Controlled Room Temperature
Type Intravenous
Volume 20 mL
Latex Information indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system$9.50