Subtotal:
$650.00
BOTOX® Cosmetic (onabotulinumtoxinA)
Only BOTOX® Cosmetic is FDA-approved to temporarily make moderate to severe frown lines, crow’s feet, and forehead lines look better in adults.
ABOUT BOTOX® COSMETIC



CLINICALLY PROVEN, RELIABLE RESULTS FOR
FOREHEAD LINES2
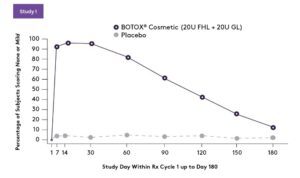
PRIMARY EFFICACY END POINT: Composite investigator and subject assessment of forehead line severity at maximum eyebrow elevation at day 30-responder rates (% and number of subjects achieving a ≥ 2-grade improvement on Facial Wrinkle Scale [FWS] from baseline)2

SECONDARY EFFICACY END POINT: Investigator-assessed achievement of none or mild from baseline on FWS at maximum eyebrow elevation (treatment success)2
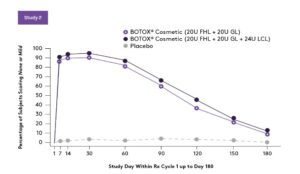
SIMILAR RESULTS FOR FOREHEAD LINES
Response rate for forehead lines across multiple treatment cycles is similar. A total of 165 and 197 patients received 3 cycles of BOTOX® Cosmetic over 1 year, 40 Units (20 Units for forehead lines with 20 Units for glabellar lines) and 64 Units (20 Units for forehead lines, 20 Units for glabellar lines, and 24 Units for lateral canthal lines), respectively.2
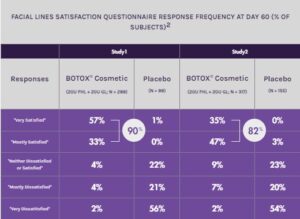
BOTOX® COSMETIC PROVIDES PATIENT SATISFACTION
90%
of subjects in Study 1 reported being “Very Satisfied” or “Mostly Satisfied” with BOTOX® Cosmetic compared to 1% with placebo at day 602
82%
of subjects in Study 2 reported being “Very Satisfied” or “Mostly Satisfied” with BOTOX® Cosmetic compared to 3% with placebo at day 602
THE ONE LIKE NO OTHER—
FROM PROCESS TO PATIENT2,10
There’s nothing else like BOTOX® Cosmetic (onabotulinumtoxinA); it’s impossible to exactly replicate. This is because critical quality attributes define the complex fingerprint of a biologic such as BOTOX®.2,10 Just as no two fingerprints are the same, no two neurotoxins are the same.10,11


WHY BOTOX® COSMETIC?






*92% of patients (n = 142) reported they discussed BOTOX® Cosmetic the first time they were treated. 85% of patients (n = 314) reported they are likely to continue using BOTOX® Cosmetic. Results from online market research surveys. Based on neurotoxin and/or filler patients.5,6REFERENCES: 1. Brin MF, James C, Maltman J. Botulinum toxin type A products are not interchangeable: a review of the evidence. Biologics. 2014;8:227-241 2. Data on file, Allergan, October 29, 2020; Westport Manufacturing Process. 3. BOTOX® Cosmetic Prescribing Information, July 2020. 4. Data on file, Allergan, July 2018; BOTOX® Cosmetic Module (UN11). 5. Data on file, Allergan, March 27, 2020; Neurotoxin Consumer ATU – Final Report. Tracker. 6. Data on file, Allergan, 2016; Facial Injectables Neurotoxins Consumer A&U Tracker.
RESULTS

BOTOX® Cosmetic is the one that patients ask for, come back for, and tell others about.2,*
*92% of patients (n = 342) report they discussed BOTOX® Cosmetic the first time they were treated. The majority of patients (85%; n = 314) are likely to continue using BOTOX® Cosmetic. Nearly 9 in 10 BOTOX® Cosmetic patients (n = 314) are willing to tell others about their treatment. Results from an online market research survey. Based on neurotoxin patients.
30-DAY RESULTS
Real patient treated for the appearance of moderate to severe forehead lines, lateral canthal lines, and glabellar lines. Results may vary.
Patients were evaluated by their specialist at least 3 months after their first treatment and considered appropriate for retreatment in 3 areas with 64 Units.
Forehead lines
Photos taken at maximum eyebrow elevation before and after treatment with BOTOX® Cosmetic at day 30. In clinical trials at day 30, 61% (178/290) and 46% (145/318) of patients demonstrated a ≥ 2-grade improvement from baseline in forehead line severity at maximum eyebrow elevation as compared to 0% (0/101) and 1% (1/156) in placebo, as assessed by both investigators and subjects.1
Lateral canthal lines
Photos taken at maximum smile before and after treatment with BOTOX® Cosmetic at day 30. In clinical trials at day 30, 26.1% (58/222) and 20.3% (62/306) of patients demonstrated a ≥ 2-grade improvement from baseline in lateral canthal line severity at maximum smile as compared to 1.3% (3/223) and 0% (0/306) in placebo, as assessed by both investigators and subjects.
Glabellar lines
Photos taken at maximum frown before and after treatment with BOTOX® Cosmetic at day 30. In clinical trials at day 7 and day 30 as assessed by investigators, 74% (299/405) and 80% (325/405) of patients, respectively, demonstrated none or mild glabellar line severity at maximum frown as compared to 6% (8/132) and 3% (4/132) in placebo. In clinical trials at day 7 and day 30 as evaluated by patients, 82% (334/405) and 89% (362/405) of patients, respectively, achieved at least a moderate improvement in their glabellar line appearance compared to 9% (12/132) and 7% (9/132) in placebo
BOTOX® Cosmetic (onabotulinumtoxinA) IMPORTANT SAFETY INFORMATION, INCLUDING BOXED WARNING
WARNING: DISTANT SPREAD OF TOXIN EFFECT
Postmarketing reports indicate that the effects of BOTOX® Cosmetic and all botulinum toxin products may spread from the area of injection to produce symptoms consistent with botulinum toxin effects. These may include asthenia, generalized muscle weakness, diplopia, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence, and breathing difficulties. These symptoms have been reported hours to weeks after injection. Swallowing and breathing difficulties can be life threatening and there have been reports of death. The risk of symptoms is probably greatest in children treated for spasticity, but symptoms can also occur in adults treated for spasticity and other conditions, particularly in those patients who have an underlying condition that would predispose them to these symptoms. In unapproved uses and approved indications, cases of spread of effect have been reported at doses comparable to those used to treat cervical dystonia and spasticity and at lower doses.
Important Information
Indications
BOTOX® Cosmetic (onabotulinumtoxinA) is indicated in adult patients for the temporary improvement in the appearance of:
- – Moderate to severe glabellar lines associated with corrugator and/or procerus muscle activity
- – Moderate to severe lateral canthal lines associated with orbicularis oculi activity
- – Moderate to severe forehead lines associated with frontalis activity
IMPORTANT SAFETY INFORMATION (continued)
CONTRAINDICATIONS
BOTOX® Cosmetic is contraindicated in the presence of infection at the proposed injection site(s) and in individuals with known hypersensitivity to any botulinum toxin preparation or to any of the components in the formulation.
WARNINGS AND PRECAUTIONS
Lack of Interchangeability Between Botulinum Toxin Products
The potency Units of BOTOX® Cosmetic are specific to the preparation and assay method utilized. They are not interchangeable with other preparations of botulinum toxin products and, therefore, units of biological activity of BOTOX® Cosmetic cannot be compared to nor converted into units of any other botulinum toxin products assessed with any other specific assay method.
Spread of Toxin Effect
Please refer to Boxed Warning for Distant Spread of Toxin Effect.
No definitive serious adverse event reports of distant spread of toxin effect associated with dermatologic use of BOTOX® Cosmetic at the labeled dose of 20 Units (for glabellar lines), 24 Units (for lateral canthal lines), 40 Units (for forehead lines with glabellar lines), 44 Units (for simultaneous treatment of lateral canthal lines and glabellar lines), and 64 Units (for simultaneous treatment of lateral canthal lines, glabellar lines, and forehead lines) have been reported. Patients or caregivers should be advised to seek immediate medical care if swallowing, speech, or respiratory disorders occur.
Serious Adverse Reactions With Unapproved Use
Serious adverse reactions, including excessive weakness, dysphagia, and aspiration pneumonia, with some adverse reactions associated with fatal outcomes, have been reported in patients who received BOTOX® injections for unapproved uses. In these cases, the adverse reactions were not necessarily related to distant spread of toxin, but may have resulted from the administration of BOTOX® to the site of injection and/or adjacent structures. In several of the cases, patients had pre-existing dysphagia or other significant disabilities. There is insufficient information to identify factors associated with an increased risk for adverse reactions associated with the unapproved uses of BOTOX®. The safety and effectiveness of BOTOX® for unapproved uses have not been established.
Hypersensitivity Reactions
Serious and/or immediate hypersensitivity reactions have been reported. These reactions include anaphylaxis, serum sickness, urticaria, soft-tissue edema, and dyspnea. If such reactions occur, further injection of BOTOX® Cosmetic should be discontinued and appropriate medical therapy immediately instituted. One fatal case of anaphylaxis has been reported in which lidocaine was used as the diluent and, consequently, the causal agent cannot be reliably determined.
Cardiovascular System
There have been reports following administration of BOTOX® of adverse events involving the cardiovascular system, including arrhythmia and myocardial infarction, some with fatal outcomes. Some of these patients had risk factors including pre-existing cardiovascular disease. Use caution when administering to patients with pre-existing cardiovascular disease.
Increased Risk of Clinically Significant Effects With Pre-existing Neuromuscular Disorders
Individuals with peripheral motor neuropathic diseases, amyotrophic lateral sclerosis, or neuromuscular junction disorders (eg, myasthenia gravis or Lambert-Eaton syndrome) should be monitored when given botulinum toxin. Patients with neuromuscular disorders may be at increased risk of clinically significant effects including generalized muscle weakness, diplopia, ptosis, dysphonia, dysarthria, severe dysphagia, and respiratory compromise from onabotulinumtoxinA (see Warnings and Precautions).
Dysphagia and Breathing Difficulties
Treatment with BOTOX® and other botulinum toxin products can result in swallowing or breathing difficulties. Patients with pre-existing swallowing or breathing difficulties may be more susceptible to these complications. In most cases, this is a consequence of weakening of muscles in the area of injection that are involved in breathing or oropharyngeal muscles that control swallowing or breathing (see Boxed Warning).
Pre-existing Conditions at the Injection Site
Caution should be used when BOTOX® Cosmetic treatment is used in the presence of inflammation at the proposed injection site(s) or when excessive weakness or atrophy is present in the target muscle(s).
Dry Eye in Patients Treated With BOTOX® Cosmetic
There have been reports of dry eye associated with BOTOX® Cosmetic injection in or near the orbicularis oculi muscle. If symptoms of dry eye (eg, eye irritation, photophobia, or visual changes) persist, consider referring patients to an ophthalmologist.
Human Albumin and Transmission of Viral Diseases
This product contains albumin, a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases and variant Creutzfeldt-Jakob disease (vCJD). There is a theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD), but if that risk actually exists, the risk of transmission would also be considered extremely remote. No cases of transmission of viral diseases, CJD or vCJD have ever been identified for licensed albumin or albumin contained in other licensed products.
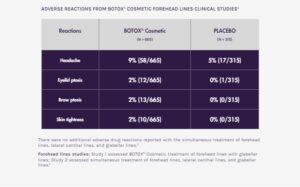
ADVERSE REACTIONS
The most frequently reported adverse reactions following injection of BOTOX® Cosmetic for glabellar lines were eyelid ptosis (3%), facial pain (1%), facial paresis (1%), and muscular weakness (1%).
The most frequently reported adverse reaction following injection of BOTOX® Cosmetic for lateral canthal lines was eyelid edema (1%).
The most frequently reported adverse reactions following injection of BOTOX® Cosmetic for forehead lines with glabellar lines were headache (9%), brow ptosis (2%), and eyelid ptosis (2%).
DRUG INTERACTIONS
Co-administration of BOTOX® Cosmetic and aminoglycosides or other agents interfering with neuromuscular transmission (eg, curare-like compounds) should only be performed with caution as the effect of the toxin may be potentiated. Use of anticholinergic drugs after administration of BOTOX® Cosmetic may potentiate systemic anticholinergic effects.
The effect of administering different botulinum neurotoxin products at the same time or within several months of each other is unknown. Excessive neuromuscular weakness may be exacerbated by administration of another botulinum toxin prior to the resolution of the effects of a previously administered botulinum toxin.
Excessive weakness may also be exaggerated by administration of a muscle relaxant before or after administration of BOTOX® Cosmetic.
USE IN SPECIFIC POPULATIONS
There are no studies or adequate data from postmarketing surveillance on the developmental risk associated with use of BOTOX® Cosmetic in pregnant women. There are no data on the presence of BOTOX® Cosmetic in human or animal milk, the effects on the breastfed child, or the effects on milk production.